Label: TECVAYLI- teclistamab injection
- NDC Code(s): 57894-449-01, 57894-450-01
- Packager: Janssen Biotech, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated August 26, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use TECVAYLI safely and effectively. See full prescribing information for TECVAYLI.
TECVAYLI ®(teclistamab-cqyv) injection, for subcutaneous use
Initial U.S. Approval: 2022WARNING: CYTOKINE RELEASE SYNDROME and NEUROLOGIC TOXICITY including IMMUNE EFFECTOR CELL-ASSOCIATED NEUROTOXICITY SYNDROME
See full prescribing information for complete boxed warning.
Cytokine release syndrome (CRS), including life-threatening or fatal reactions, can occur in patients receiving TECVAYLI. Initiate treatment with TECVAYLI step-up dosing schedule to reduce risk of CRS. Withhold TECVAYLI until CRS resolves or permanently discontinue based on severity. ( 2.1, 2.4, 5.1)
Neurologic toxicity, including Immune Effector Cell-Associated Neurotoxicity Syndrome (ICANS) and serious, life-threatening or fatal reactions, can occur in patients receiving TECVAYLI. Monitor patients for signs or symptoms of neurologic toxicity, including ICANS, during treatment. Withhold TECVAYLI until neurologic toxicity resolves or permanently discontinue based on severity. ( 2.4, 5.2)
TECVAYLI is available only through a restricted program called the TECVAYLI and TALVEY Risk Evaluation and Mitigation Strategy (REMS). ( 5.3)
RECENT MAJOR CHANGES
Boxed Warnings 8/2025 Dosage and Administration, Recommended Dosage ( 2.1) 2/2024 Dosage and Administration, Restarting TECVAYLI after Dosage Delay ( 2.3) 11/2024 Dosage and Administration, Preparation and Administration ( 2.5) 2/2024 Warnings and Precautions, Neurologic Toxicity including ICANS ( 5.2) 8/2025 INDICATIONS AND USAGE
TECVAYLI is a bispecific B-cell maturation antigen (BCMA)-directed CD3 T-cell engager indicated for the treatment of adult patients with relapsed or refractory multiple myeloma who have received at least four prior lines of therapy, including a proteasome inhibitor, an immunomodulatory agent and an anti-CD38 monoclonal antibody.
This indication is approved under accelerated approval based on response rate. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial(s). ( 1)
DOSAGE AND ADMINISTRATION
TECVAYLI Recommended Dosing Schedule ( 2.1) Dosing Schedule Day Dose - *
- Step-up dose 2 may be given between 2 to 4 days after step-up dose 1 and may be given up to 7 days after step-up dose 1 to allow for resolution of adverse reactions.
- †
- First treatment dose may be given between 2 to 4 days after step-up dose 2 and may be given up to 7 days after step-up dose 2 to allow for resolution of adverse reactions.
All Patients Step-up Dosing Schedule Day 1 Step-up dose 1 0.06 mg/kg Day 4 * Step-up dose 2 0.3 mg/kg Day 7 † First treatment dose 1.5 mg/kg Weekly Dosing Schedule
One week after first treatment dose and weekly thereafter Subsequent treatment doses 1.5 mg/kg once weekly Patients who have achieved and maintained a complete response or better for a minimum of 6 months Biweekly (every two weeks) dosing schedule The dosing frequency may be decreased to 1.5 mg/kg every two weeks - For subcutaneous injection only. ( 2.1)
- Patients should be hospitalized for 48 hours after administration of all doses within the TECVAYLI step-up dosing schedule. ( 2.1)
- Administer pretreatment medications as recommended. ( 2.2)
- Refer to Tables 7, 8, and 9 to determine the dosage based on predetermined weight ranges. ( 2.5)
- See Full Prescribing Information for instructions on preparation and administration. ( 2.5)
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
None. ( 4)
WARNINGS AND PRECAUTIONS
- Hepatotoxicity: Can cause hepatotoxicity, including fatalities. Monitor liver enzymes and bilirubin at baseline and during treatment as clinically indicated. ( 5.4)
- Infections: Can cause severe, life-threatening, or fatal infections. Monitor patients for signs and symptoms of infection and treat appropriately. Withhold in patients with active infection during the step-up dosing schedule. ( 2.4, 5.5)
- Neutropenia: Monitor complete blood cell counts at baseline and periodically during treatment. ( 5.6)
- Hypersensitivity and Other Administration Reactions: Systemic administration-related reactions and local injection site reactions can occur. Withhold or consider permanent discontinuation based on severity. ( 2.4, 5.7)
- Embryo-Fetal Toxicity: May cause fetal harm. Advise females of reproductive potential of the potential risk to the fetus and to use effective contraception. ( 5.8, 8.1, 8.3)
ADVERSE REACTIONS
The most common adverse reactions (≥20%) are pyrexia, cytokine release syndrome, musculoskeletal pain, injection site reaction, fatigue, upper respiratory tract infection, nausea, headache, pneumonia, and diarrhea.
The most common Grade 3 to 4 laboratory abnormalities (≥20%) are decreased lymphocytes, decreased neutrophils, decreased white blood cells, decreased hemoglobin, and decreased platelets. ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Janssen Biotech, Inc. at 1-800-526-7736 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 8/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: CYTOKINE RELEASE SYNDROME and NEUROLOGIC TOXICITY including IMMUNE EFFECTOR CELL-ASSOCIATED NEUROTOXICITY SYNDROME
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Recommended Pretreatment Medications
2.3 Restarting TECVAYLI after Dosage Delay
2.4 Dosage Modifications for Adverse Reactions
2.5 Preparation and Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Cytokine Release Syndrome
5.2 Neurologic Toxicity including ICANS
5.3 TECVAYLI and TALVEY REMS
5.4 Hepatotoxicity
5.5 Infections
5.6 Neutropenia
5.7 Hypersensitivity and Other Administration Reactions
5.8 Embryo-Fetal Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.6 Immunogenicity
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: CYTOKINE RELEASE SYNDROME and NEUROLOGIC TOXICITY including IMMUNE EFFECTOR CELL-ASSOCIATED NEUROTOXICITY SYNDROME
Cytokine release syndrome (CRS), including life-threatening or fatal reactions, can occur in patients receiving TECVAYLI. Initiate treatment with TECVAYLI step-up dosing schedule to reduce risk of CRS. Withhold TECVAYLI until CRS resolves or permanently discontinue based on severity [see Dosage and Administration (2.1, 2.4)and Warnings and Precautions (5.1)] .
Neurologic toxicity, including Immune Effector Cell-Associated Neurotoxicity Syndrome (ICANS) and serious, life-threatening or fatal reactions, can occur with TECVAYLI. Monitor patients for signs or symptoms of neurologic toxicity, including ICANS, during treatment. Withhold TECVAYLI until neurologic toxicity resolves or permanently discontinue based on severity [see Dosage and Administration (2.4)and Warnings and Precautions (5.2)] .
Because of the risk of CRS and neurologic toxicity, including ICANS, TECVAYLI is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the TECVAYLI and TALVEY REMS [see Warnings and Precautions (5.3)] .
-
1 INDICATIONS AND USAGE
TECVAYLI is indicated for the treatment of adult patients with relapsed or refractory multiple myeloma who have received at least four prior lines of therapy, including a proteasome inhibitor, an immunomodulatory agent and an anti-CD38 monoclonal antibody.
This indication is approved under accelerated approval based on response rate [see Clinical Studies (14)] . Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trial(s).
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
For subcutaneous injection only.
The recommended dosing schedule for TECVAYLI is provided in Table 1. The recommended dosage of TECVAYLI is step-up doses of 0.06 mg/kg and 0.3 mg/kg followed by 1.5 mg/kg once weekly until disease progression or unacceptable toxicity. In patients who have achieved and maintained a complete response or better for a minimum of 6 months, the dosing frequency may be decreased to 1.5 mg/kg every two weeks until disease progression or unacceptable toxicity.
Administer pretreatment medications prior to each dose of the TECVAYLI step-up dosing schedule, which includes step-up dose 1, step-up dose 2, and the first treatment dose as described in Table 1 [see Dosage and Administration (2.2)] .
Administer TECVAYLI subcutaneously according to the step-up dosing schedule in Table 1 to reduce the incidence and severity of cytokine release syndrome (CRS). Due to the risk of CRS and neurologic toxicity, including ICANS, patients should be hospitalized for 48 hours after administration of all doses within the TECVAYLI step-up dosing schedule [see Dosage and Administration (2.4)and Warnings and Precautions (5.1, 5.2)] .
Table 1: TECVAYLI Dosing Schedule Dosing schedule Day Dose - *
- See Table 2 for recommendations on restarting TECVAYLI after dose delays [see Dosage and Administration (2.3)].
- †
- Step-up dose 2 may be given between 2 to 4 days after step-up dose 1 and may be given up to 7 days after step-up dose 1 to allow for resolution of adverse reactions.
- ‡
- First treatment dose may be given between 2 to 4 days after step-up dose 2 and may be given up to 7 days after step-up dose 2 to allow for resolution of adverse reactions.
All Patients Step-up dosing schedule * Day 1 Step-up dose 1 0.06 mg/kg Day 4 † Step-up dose 2 0.3 mg/kg Day 7 ‡ First treatment dose 1.5 mg/kg Weekly dosing schedule * One week after first treatment dose and weekly thereafter Subsequent treatment doses 1.5 mg/kg once weekly Patients who have achieved and maintained a complete response or better for a minimum of 6 months Biweekly (every two weeks) dosing schedule * The dosing frequency may be decreased to 1.5 mg/kg every two weeks. Refer to Tables 7, 8, and 9 to determine the dosage based on predetermined weight ranges [see Dosage and Administration (2.5)] .
2.2 Recommended Pretreatment Medications
Administer the following pretreatment medications 1 to 3 hours before each dose of the TECVAYLI step-up dosing schedule, which includes step-up dose 1, step-up dose 2, and the first treatment dose (see Table 1), to reduce the risk of CRS [see Warnings and Precautions (5.1)and Adverse Reactions (6.1)] .
- Corticosteroid (oral or intravenous dexamethasone 16 mg)
- Histamine-1 (H1) receptor antagonist (oral or intravenous diphenhydramine 50 mg or equivalent)
- Antipyretics (oral or intravenous acetaminophen 650 mg to 1,000 mg or equivalent)
Administration of pretreatment medications may be required prior to administration of subsequent doses of TECVAYLI in the following patients:
- Patients who repeat doses within the TECVAYLI step-up dosing schedule following a dose delay [see Dosage and Administration (2.3)].
- Patients who experienced CRS following the prior dose of TECVAYLI [see Dosage and Administration (2.4)].
2.3 Restarting TECVAYLI after Dosage Delay
If a dose of TECVAYLI is delayed, restart therapy based on the recommendations in Table 2 and resume the treatment schedule accordingly [see Dosage and Administration (2.1)] . Administer pretreatment medications as indicated in Table 2 .Due to the risk of CRS and neurologic toxicity, including ICANS, patients should be hospitalized for 48 hours after administration of all doses within the TECVAYLI step-up dosing schedule [see Dosage and Administration (2.4)and Warnings and Precautions (5.1, 5.2)].
Table 2: Recommendations for Restarting Therapy with TECVAYLI After Dose Delay Last dose administered Time since the last dose administered Action Step-up dose 1 More than 7 days Restart TECVAYLI step-up dosing schedule at step-up dose 1 (0.06 mg/kg). * Step-up dose 2 8 days to 28 days Repeat step-up dose 2 (0.3 mg/kg) *and continue TECVAYLI step-up dosing schedule. More than 28 days † Restart TECVAYLI step-up dosing schedule at step-up dose 1 (0.06 mg/kg). * Any weekly treatment dose 28 days or less Continue TECVAYLI at last treatment dose in weekly schedule (1.5 mg/kg once weekly; see Table 1). 29 days to 56 days † Restart TECVAYLI step-up dosing schedule at step-up dose 2 (0.3 mg/kg). * More than 56 days † Restart TECVAYLI step-up dosing schedule at step-up dose 1 (0.06 mg/kg). * Any biweekly (every two weeks) treatment dose 63 days or less † Continue TECVAYLI at last treatment dose in biweekly schedule (1.5 mg/kg every two weeks; see Table 1). 64 days to 112 days † Restart TECVAYLI step-up dosing schedule at step up dose 2 (0.3 mg/kg). * More than 112 days † Restart TECVAYLI step-up dosing schedule at step-up dose 1 (0.06 mg/kg). * 2.4 Dosage Modifications for Adverse Reactions
Dosage reductions of TECVAYLI are not recommended.
Dosage delays may be required to manage toxicities related to TECVAYLI [see Warnings and Precautions (5)].
See Tables 3, 4, and 5for recommended actions for adverse reactions of CRS, neurologic toxicity, and ICANS. See Table 6for recommended actions for other adverse reactions following administration of TECVAYLI.
Management of CRS, Neurologic Toxicity and ICANS
Cytokine Release Syndrome (CRS)
Management recommendations for CRS are summarized in Table 3.
Identify CRS based on clinical presentation [see Warnings and Precautions (5.1)]. Evaluate and treat other causes of fever, hypoxia, and hypotension.
If CRS is suspected, withhold TECVAYLI until CRS resolves. Manage according to the recommendations in Table 3 and consider further management per current practice guidelines. Administer supportive therapy for CRS, which may include intensive care for severe or life-threatening CRS. Consider laboratory testing to monitor for disseminated intravascular coagulation (DIC), hematology parameters, as well as pulmonary, cardiac, renal, and hepatic function.
Table 3: Recommendations for Management of Cytokine Release Syndrome Grade * Presenting Symptoms Actions - *
- Based on American Society for Transplantation and Cellular Therapy (ASTCT) 2019 grading for CRS.
- †
- Attributed to CRS. Fever may not always be present concurrently with hypotension or hypoxia as it may be masked by interventions such as antipyretics or anticytokine therapy.
- ‡
- See Table 2 for recommendations on restarting TECVAYLI after dose delays [see Dosage and Administration (2.3)].
- §
- Low-flow nasal cannula is ≤6 L/min, and high-flow nasal cannula is >6 L/min.
Grade 1 Temperature ≥100.4 °F (38 °C) † - Withhold TECVAYLI until CRS resolves.
- Administer pretreatment medications prior to next dose of TECVAYLI. ‡
Grade 2 Temperature ≥100.4 °F (38 °C) †with:
Hypotension responsive to fluids and not requiring vasopressors,
and/or,
Oxygen requirement of low-flow nasal cannula §or blow-by.- Withhold TECVAYLI until CRS resolves.
- Administer pretreatment medications prior to next dose of TECVAYLI. ‡
- Patients should be hospitalized for 48 hours following the next dose of TECVAYLI [see Dosage and Administration (2.1)]. ‡
Grade 3 Temperature ≥100.4 °F (38 °C) †with:
Hypotension requiring one vasopressor with or without vasopressin,
and/or,
Oxygen requirement of high-flow nasal cannula §, facemask, non-rebreather mask, or Venturi mask.First Occurrence of Grade 3 CRS with Duration Less than 48 Hours: - Withhold TECVAYLI until CRS resolves.
- Provide supportive therapy, which may include intensive care.
- Administer pretreatment medications prior to next dose of TECVAYLI. ‡
- Patients should be hospitalized for 48 hours following the next dose of TECVAYLI [see Dosage and Administration (2.1)]. ‡
Recurrent Grade 3 CRS or Grade 3 CRS with Duration 48 Hours or Longer: - Permanently discontinue TECVAYLI.
- Provide supportive therapy, which may include intensive care.
Grade 4 Temperature ≥100.4 °F (38 °C) †with:
Hypotension requiring multiple vasopressors (excluding vasopressin),
and/or,
Oxygen requirement of positive pressure (e.g., continuous positive airway pressure (CPAP), bilevel positive airway pressure (BiPAP), intubation, and mechanical ventilation).- Permanently discontinue TECVAYLI.
- Provide supportive therapy, which may include intensive care.
Neurologic Toxicity and ICANS
Management recommendations for neurologic toxicity and ICANS are summarized in Tables 4 and 5.
At the first sign of neurologic toxicity, including ICANS, withhold TECVAYLI and consider neurology evaluation. Rule out other causes of neurologic symptoms. Provide supportive therapy, which may include intensive care, for severe or life-threatening neurologic toxicities, including ICANS [see Warnings and Precautions (5.2)] . Manage ICANS according to the recommendations in Table 5 and consider further management per current practice guidelines.
Table 4: Recommendations for Management of Neurologic Toxicity (excluding ICANS) Adverse Reaction Severity * Actions Neurologic Toxicity *(excluding ICANS) Grade 1 - Withhold TECVAYLI until neurologic toxicity symptoms resolve or stabilize. †
Grade 2
Grade 3 (First occurrence)- Withhold TECVAYLI until neurologic toxicity symptoms improve to Grade 1 or less. †
- Provide supportive therapy.
Grade 3 (Recurrent)
Grade 4- Permanently discontinue TECVAYLI.
- Provide supportive therapy, which may include intensive care.
Table 5: Recommendations for Management of Immune Effector Cell-Associated Neurotoxicity Syndrome Grade * Presenting Symptoms † Actions - *
- Based on American Society for Transplantation and Cellular Therapy (ASTCT) 2019 grading for ICANS.
- †
- Management is determined by the most severe event, not attributable to any other cause.
- ‡
- If patient is arousable and able to perform Immune Effector Cell-Associated Encephalopathy (ICE) Assessment, assess: Orientation (oriented to year, month, city, hospital = 4 points); Naming (name 3 objects, e.g., point to clock, pen, button = 3 points); Following Commands (e.g., "show me 2 fingers" or "close your eyes and stick out your tongue" = 1 point); Writing (ability to write a standard sentence = 1 point; and Attention (count backwards from 100 by ten = 1 point). If patient is unarousable and unable to perform ICE Assessment (Grade 4 ICANS) = 0 points.
- §
- Not attributable to any other cause.
- ¶
- See Table 2 for recommendations on restarting TECVAYLI after dose delays [see Dosage and Administration (2.3)].
- #
- All references to dexamethasone administration are dexamethasone or equivalent.
Grade 1 ICE score 7–9 ‡,
or depressed level of consciousness §: awakens spontaneously.- Withhold TECVAYLI until ICANS resolves. ¶
- Monitor neurologic symptoms and consider consultation with neurologist and other specialists for further evaluation and management, including consideration for starting non-sedating, anti-seizure medicines for seizure prophylaxis.
Grade 2 ICE score 3–6 ‡,
or depressed level of consciousness §: awakens to voice.- Withhold TECVAYLI until ICANS resolves.
- Administer dexamethasone #10 mg intravenously every 6 hours. Continue dexamethasone use until resolution to Grade 1 or less then taper.
- Monitor neurologic symptoms and consider consultation with neurologist and other specialists for further evaluation and management, including consideration for starting non-sedating, anti-seizure medicines for seizure prophylaxis.
- Patients should be hospitalized for 48 hours following the next dose of TECVAYLI [see Dosage and Administration (2.1)] . ¶
Grade 3 ICE score 0–2 ‡,
or depressed level of consciousness §: awakens only to tactile stimulus,
or seizures §, either:- any clinical seizure, focal or generalized, that resolves rapidly, or
- non-convulsive seizures on electroencephalogram (EEG) that resolve with intervention,
First Occurrence of Grade 3 ICANS: - Withhold TECVAYLI until ICANS resolves.
- Administer dexamethasone #10 mg intravenously every 6 hours. Continue dexamethasone use until resolution to Grade 1 or less, then taper.
- Monitor neurologic symptoms and consider consultation with neurologist and other specialists for further evaluation and management, including consideration for starting non-sedating, anti-seizure medicines for seizure prophylaxis.
- Provide supportive therapy, which may include intensive care.
- Patients should be hospitalized for 48 hours following the next dose of TECVAYLI [see Dosage and Administration (2.1)] . ¶
Recurrent Grade 3 ICANS: - Permanently discontinue TECVAYLI
- Administer dexamethasone #10 mg intravenously and repeat dose every 6 hours. Continue dexamethasone use until resolution to Grade 1 or less, then taper.
- Monitor neurologic symptoms and consider consultation with neurologist and other specialists for further evaluation and management, including consideration for starting non-sedating, anti-seizure medicines for seizure prophylaxis.
- Provide supportive therapy, which may include intensive care.
Grade 4 ICE score 0 ‡,
or depressed level of consciousness §: either:- patient is unarousable or requires vigorous or repetitive tactile stimuli to arouse, or
- stupor or coma,
- life-threatening prolonged seizure (>5 minutes), or
- repetitive clinical or electrical seizures without return to baseline in between,
- deep focal motor weakness such as hemiparesis or paraparesis,
- diffuse cerebral edema on neuroimaging, or
- decerebrate or decorticate posturing, or
- cranial nerve VI palsy, or
- papilledema, or
- Cushing's triad.
- Permanently discontinue TECVAYLI.
- Administer dexamethasone #10 mg intravenously and repeat dose every 6 hours. Continue dexamethasone use until resolution to Grade 1 or less, then taper.
- Alternatively, consider administration of methylprednisolone 1,000 mg per day intravenously and continue methylprednisolone 1,000 mg per day intravenously for 2 or more days.
- Monitor neurologic symptoms and consider consultation with neurologist and other specialists for further evaluation and management, including consideration for starting non-sedating, anti-seizure medicines for seizure prophylaxis.
- Provide supportive therapy, which may include intensive care.
Table 6: Recommended Dosage Modifications for Other Adverse Reactions Adverse Reactions Severity Actions Infections *[see Warnings and Precautions (5.5)] All Grades - Withhold TECVAYLI in patients with active infection during the step-up dosing schedule. †
Grade 3 - Withhold subsequent treatment doses of TECVAYLI (i.e., doses administered after TECVAYLI step-up dosing schedule) until infection improves to Grade 1 or less. †
Grade 4 - Consider permanent discontinuation of TECVAYLI.
- If TECVAYLI is not permanently discontinued, withhold subsequent treatment doses of TECVAYLI (i.e., doses administered after TECVAYLI step-up dosing schedule) until infection improves to Grade 1 or less. †
Hematologic Toxicities [see Warnings and Precautions (5.6)and Adverse Reactions (6.1)] Absolute neutrophil count less than 0.5 × 10 9/L - Withhold TECVAYLI until absolute neutrophil count is 0.5 × 10 9/L or higher. †
Febrile neutropenia - Withhold TECVAYLI until absolute neutrophil count is 1 × 10 9/L or higher and fever resolves. †
Hemoglobin less than 8 g/dL - Withhold TECVAYLI until hemoglobin is 8 g/dL or higher. †
Platelet count less than 25,000/mcL
Platelet count between 25,000/mcL and 50,000/mcL with bleeding- Withhold TECVAYLI until platelet count is 25,000/mcL or higher and no evidence of bleeding. †
Other Non-Hematologic Adverse Reactions *
[see Warnings and Precautions (5.4)and Adverse Reactions (6.1)]Grade 3 - Withhold TECVAYLI until adverse reaction improves to Grade 1 or less. †
Grade 4 - Consider permanent discontinuation of TECVAYLI.
- If TECVAYLI is not permanently discontinued, withhold subsequent treatment doses of TECVAYLI (i.e., doses administered after TECVAYLI step-up dosing schedule) until adverse reaction improves to Grade 1 or less. †
2.5 Preparation and Administration
TECVAYLI is intended for subcutaneous use by a healthcare provider only.
TECVAYLI should be administered by a healthcare provider with adequate medical personnel and appropriate medical equipment to manage severe reactions, including CRS and ICANS [see Warnings and Precautions (5.1, 5.2)] .
TECVAYLI is a clear to slightly opalescent, colorless to light yellow solution. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use if the solution is discolored, or cloudy, or if foreign particles are present.
TECVAYLI 30 mg/3 mL (10 mg/mL) vial and TECVAYLI 153 mg/1.7 mL (90 mg/mL) vial are supplied as ready-to-use solution that do not need dilution prior to administration.
Do not combine TECVAYLI vials of different concentrations to achieve treatment dose.
Use aseptic technique to prepare and administer TECVAYLI.
Preparation of TECVAYLI
Refer to the following reference tables for the preparation of TECVAYLI.
Refer to Tables 7, 8, and 9 below to determine the dosage based on predetermined weight ranges.
Use Table 7 to determine total dose, injection volume and number of vials required based on patient's actual body weight for step-up dose 1 using TECVAYLI 30 mg/3 mL (10 mg/mL) vial.
Table 7: Step-up Dose 1 (0.06 mg/kg) Injection Volumes using TECVAYLI 30 mg/3 mL (10 mg/mL) Vial Patient Body Weight
(kg)Total Dose
(mg)Volume of Injection
(mL)Number of Vials
(1 vial=3 mL)35 to 39 2.2 0.22 1 40 to 44 2.5 0.25 1 45 to 49 2.8 0.28 1 50 to 59 3.3 0.33 1 60 to 69 3.9 0.39 1 70 to 79 4.5 0.45 1 80 to 89 5.1 0.51 1 90 to 99 5.7 0.57 1 100 to 109 6.3 0.63 1 110 to 119 6.9 0.69 1 120 to 129 7.5 0.75 1 130 to 139 8.1 0.81 1 140 to 149 8.7 0.87 1 150 to 160 9.3 0.93 1 Use Table 8 to determine total dose, injection volume and number of vials required based on patient's actual body weight for step-up dose 2 using TECVAYLI 30 mg/3 mL (10 mg/mL) vial.
Table 8: Step-up Dose 2 (0.3 mg/kg) Injection Volumes using TECVAYLI 30 mg/3 mL (10 mg/mL) Vial Patient Body Weight
(kg)Total Dose
(mg)Volume of Injection
(mL)Number of Vials
(1 vial=3 mL)35 to 39 11 1.1 1 40 to 44 13 1.3 1 45 to 49 14 1.4 1 50 to 59 16 1.6 1 60 to 69 19 1.9 1 70 to 79 22 2.2 1 80 to 89 25 2.5 1 90 to 99 28 2.8 1 100 to 109 31 3.1 2 110 to 119 34 3.4 2 120 to 129 37 3.7 2 130 to 139 40 4 2 140 to 149 43 4.3 2 150 to 160 47 4.7 2 Use Table 9 to determine total dose, injection volume and number of vials required based on patient's actual body weight for the treatment dose using TECVAYLI 153 mg/1.7 mL (90 mg/mL) vial.
Table 9: Treatment Dose (1.5 mg/kg) Injection Volumes using TECVAYLI 153 mg/1.7 mL (90 mg/mL) Vial Patient Body Weight
(kg)Total Dose
(mg)Volume of Injection
(mL)Number of Vials
(1 vial=1.7 mL)35 to 39 56 0.62 1 40 to 44 63 0.7 1 45 to 49 70 0.78 1 50 to 59 82 0.91 1 60 to 69 99 1.1 1 70 to 79 108 1.2 1 80 to 89 126 1.4 1 90 to 99 144 1.6 1 100 to 109 153 1.7 1 110 to 119 171 1.9 2 120 to 129 189 2.1 2 130 to 139 198 2.2 2 140 to 149 216 2.4 2 150 to 160 234 2.6 2 Remove the appropriate strength TECVAYLI vial from refrigerated storage [2 °C to 8 °C (36 °F to 46 °F)].
Once removed from refrigerated storage, equilibrate TECVAYLI to ambient temperature [15 °C to 30 °C (59 °F to 86 °F)] for at least 15 minutes. Do not warm TECVAYLI in any other way.
Once equilibrated, gently swirl the vial for approximately 10 seconds to mix. Do not shake.
Withdraw the required injection volume of TECVAYLI from the vial(s) into an appropriately sized syringe using a transfer needle.
Each injection volume should not exceed 2 mL. Divide doses requiring greater than 2 mL equally into multiple syringes.
TECVAYLI is compatible with stainless steel injection needles and polypropylene or polycarbonate syringe material.
Replace the transfer needle with an appropriately sized needle for injection.
Administration of TECVAYLI
Inject the required volume of TECVAYLI into the subcutaneous tissue of the abdomen (preferred injection site). Alternatively, TECVAYLI may be injected into the subcutaneous tissue at other sites (e.g., thigh). If multiple injections are required, TECVAYLI injections should be at least 2 cm apart.
Do not inject into tattoos or scars or areas where the skin is red, bruised, tender, hard or not intact.
Any unused product or waste material should be disposed in accordance with local requirements.
Storage
If the prepared dosing syringe(s) of TECVAYLI is not used immediately, store syringe(s) at 2 °C to 8 °C (36 °F to 46 °F) or at ambient temperature 15 °C to 30 °C (59 °F to 86 °F) for a maximum of 20 hours. Discard syringe(s) after 20 hours, if not used.
Monitoring
Due to the risk of CRS and neurologic toxicity, including ICANS, patients should be hospitalized for 48 hours after administration of all doses within the TECVAYLI step-up dosing schedule [see Dosage and Administration (2.1)and Warnings and Precautions (5.1, 5.2)] .
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Cytokine Release Syndrome
TECVAYLI can cause cytokine release syndrome (CRS), including life-threatening or fatal reactions [see Adverse Reactions (6.1)] .
In the clinical trial, CRS occurred in 72% of patients who received TECVAYLI at the recommended dose, with Grade 1 CRS occurring in 50% of patients, Grade 2 in 21%, and Grade 3 in 0.6%. Recurrent CRS occurred in 33% of patients. Most patients experienced CRS following step-up dose 1 (42%), step-up dose 2 (35%), or the initial treatment dose (24%). Less than 3% of patients developed first occurrence of CRS following subsequent doses of TECVAYLI. The median time to onset of CRS was 2 (range: 1 to 6) days after the most recent dose with a median duration of 2 (range: 1 to 9) days.
Clinical signs and symptoms of CRS included, but were not limited to, fever, hypoxia, chills, hypotension, sinus tachycardia, headache, and elevated liver enzymes (aspartate aminotransferase and alanine aminotransferase elevation).
Initiate therapy according to TECVAYLI step-up dosing schedule to reduce risk of CRS [see Dosage and Administration (2.1, 2.4)] . Administer pretreatment medications to reduce risk of CRS and monitor patients following administration of TECVAYLI accordingly [see Dosage and Administration (2.2, 2.4)] .
At the first sign of CRS, immediately evaluate patient for hospitalization. Administer supportive care based on severity and consider further management per current practice guidelines. Withhold or permanently discontinue TECVAYLI based on severity [see Dosage and Administration (2.4)] .
TECVAYLI is available only through a restricted program under a REMS [see Warnings and Precautions (5.3)].
5.2 Neurologic Toxicity including ICANS
TECVAYLI can cause serious, life-threatening or fatal neurologic toxicity, including Immune Effector Cell-Associated Neurotoxicity Syndrome (ICANS) [see Adverse Reactions (6.1)] .
In the clinical trial, neurologic toxicity occurred in 57% of patients who received TECVAYLI at the recommended dose, with Grade 3 or 4 neurologic toxicity occurring in 2.4% of patients. The most frequent neurologic toxicities were headache (25%), motor dysfunction (16%), sensory neuropathy (15%), and encephalopathy (13%).
With longer follow-up, Grade 4 seizure and fatal Guillain-Barré syndrome (one patient each) occurred in patients who received TECVAYLI.
In the clinical trial, ICANS was reported in 6% of patients who received TECVAYLI at the recommended dose [see Adverse Reactions (6.1)] . Recurrent ICANS occurred in 1.8% of patients. Most patients experienced ICANS following step-up dose 1 (1.2%), step-up dose 2 (0.6%), or the initial treatment dose (1.8%). Less than 3% of patients developed first occurrence of ICANS following subsequent doses of TECVAYLI. The median time to onset of ICANS was 4 (range: 2 to 8) days after the most recent dose with a median duration of 3 (range: 1 to 20) days. The most frequent clinical manifestations of ICANS reported were confusional state and dysgraphia. The onset of ICANS can be concurrent with CRS, following resolution of CRS, or in the absence of CRS.
Monitor patients for signs and symptoms of neurologic toxicity during treatment. At the first sign of neurologic toxicity, including ICANS, immediately evaluate patient and provide supportive therapy based on severity. Withhold or permanently discontinue TECVAYLI based on severity per recommendations and consider further management per current practice guidelines [see Dosage and Administration (2.4)] .
Due to the potential for neurologic toxicity, patients receiving TECVAYLI are at risk of depressed level of consciousness [see Adverse Reactions (6.1)] . Advise patients to refrain from driving or operating heavy or potentially dangerous machinery during and for 48 hours after completion of TECVAYLI step-up dosing schedule and in the event of new onset of any neurologic toxicity symptoms until neurologic toxicity resolves [see Dosage and Administration (2.1)] .
TECVAYLI is available only through a restricted program under a REMS [see Warnings and Precautions (5.3)].
5.3 TECVAYLI and TALVEY REMS
TECVAYLI is available only through a restricted program under a REMS called the TECVAYLI and TALVEY REMS because of the risks of CRS and neurologic toxicity, including ICANS [see Warnings and Precautions (5.1, 5.2)].
Notable requirements of the TECVAYLI and TALVEY REMS include the following:
- Prescribers must be certified with the program by enrolling and completing training.
- Prescribers must counsel patients receiving TECVAYLI about the risk of CRS and neurologic toxicity, including ICANS, and provide patients with Patient Wallet Card.
- Pharmacies and healthcare settings that dispense TECVAYLI must be certified with the TECVAYLI and TALVEY REMS program and must verify prescribers are certified through the TECVAYLI and TALVEY REMS program.
- Wholesalers and distributers must only distribute TECVAYLI to certified pharmacies or healthcare settings.
Further information about the TECVAYLI and TALVEY REMS program is available at www.TEC-TALREMS.com or by telephone at 1-855-810-8064.
5.4 Hepatotoxicity
TECVAYLI can cause hepatotoxicity, including fatalities. In patients who received TECVAYLI at the recommended dose in the clinical trial, there was one fatal case of hepatic failure. Elevated aspartate aminotransferase (AST) occurred in 34% of patients, with Grade 3 or 4 elevations in 1.2%. Elevated alanine aminotransferase (ALT) occurred in 28% of patients, with Grade 3 or 4 elevations in 1.8%. Elevated total bilirubin occurred in 6% of patients with Grade 3 or 4 elevations in 0.6%. Liver enzyme elevation can occur with or without concurrent CRS.
Monitor liver enzymes and bilirubin at baseline and during treatment as clinically indicated. Withhold TECVAYLI or consider permanent discontinuation of TECVAYLI based on severity [see Dosage and Administration (2.4)].
5.5 Infections
TECVAYLI can cause severe, life-threatening, or fatal infections. In patients who received TECVAYLI at the recommended dose in the clinical trial, serious infections, including opportunistic infections, occurred in 30% of patients, with Grade 3 or 4 infections in 35%, and fatal infections in 4.2% [see Adverse Reactions (6.1)] .
Monitor patients for signs and symptoms of infection prior to and during treatment with TECVAYLI and treat appropriately. Administer prophylactic antimicrobials according to guidelines [see Dosage and Administration (2.2)] .
Withhold TECVAYLI or consider permanent discontinuation of TECVAYLI based on severity [see Dosage and Administration (2.4)] .
Monitor immunoglobulin levels during treatment with TECVAYLI and treat according to guidelines, including infection precautions and antibiotic or antiviral prophylaxis [see Dosage and Administration (2.2)] .
5.6 Neutropenia
TECVAYLI can cause neutropenia and febrile neutropenia. In patients who received TECVAYLI at the recommended dose in the clinical trial, decreased neutrophils occurred in 84% of patients, with Grade 3 or 4 decreased neutrophils in 56%. Febrile neutropenia occurred in 3% of patients [see Adverse Reactions (6.1)].
Monitor complete blood cell counts at baseline and periodically during treatment and provide supportive care per local institutional guidelines.
Monitor patients with neutropenia for signs of infection.
Withhold TECVAYLI based on severity [see Dosage and Administration (2.4)] .
5.7 Hypersensitivity and Other Administration Reactions
TECVAYLI can cause both systemic administration-related reactions and local injection-site reactions.
Systemic Reactions
In patients who received TECVAYLI at the recommended dose in the clinical trial, 1.2% of patients experienced systemic-administration reactions, which included Grade 1 recurrent pyrexia and Grade 1 swollen tongue.
Local Reactions
In patients who received TECVAYLI at the recommended dose in the clinical trial, injection-site reactions occurred in 35% of patients with Grade 1 injection-site reactions in 30% and Grade 2 in 4.8%.
Withhold TECVAYLI or consider permanent discontinuation of TECVAYLI based on severity [see Dosage and Administration (2.4)] .
5.8 Embryo-Fetal Toxicity
Based on its mechanism of action, TECVAYLI may cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to the fetus. Advise females of reproductive potential to use effective contraception during treatment with TECVAYLI and for 5 months after the last dose [see Use in Specific Populations (8.1, 8.3)] .
-
6 ADVERSE REACTIONS
The following adverse reactions are also described elsewhere in the labeling:
- Cytokine Release Syndrome [see Warnings and Precautions (5.1)]
- Neurologic Toxicity including ICANS [see Warnings and Precautions (5.2)]
- Hepatotoxicity [see Warnings and Precautions (5.4)]
- Infections [see Warnings and Precautions (5.5)]
- Neutropenia [see Warnings and Precautions (5.6)]
- Hypersensitivity and Other Administration Reactions [see Warnings and Precautions (5.7)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Relapsed/Refractory Multiple Myeloma
MajesTEC-1
The safety of TECVAYLI was evaluated in MajesTEC-1 [see Clinical Studies (14)] which included adult patients with relapsed or refractory multiple myeloma. Patients received step-up doses of 0.06 mg/kg and 0.3 mg/kg of TECVAYLI followed by TECVAYLI 1.5 mg/kg, subcutaneously once weekly (N=165). Among patients who received TECVAYLI, 47% were exposed for 6 months or longer and 7% were exposed for one year or longer.
The median age of patients who received TECVAYLI was 64 years (range: 33 to 84 years); 58% were male; 81% were White, 13% were Black or African American, and 2% were Asian.
Serious adverse reactions occurred in 54% of patients who received TECVAYLI. Serious adverse reactions in >2% of patients included pneumonia (15%), cytokine release syndrome (8%), sepsis (6%), general physical health deterioration (6%), COVID-19 (6%), acute kidney injury (4.8%), pyrexia (4.8%), musculoskeletal pain (2.4%), and encephalopathy (2.4%).
Fatal adverse reactions occurred in 5% of patients who received TECVAYLI, including COVID-19 (1.8%), pneumonia (1.8%), septic shock (0.6%), acute renal failure (0.6%), and hemoperitoneum (0.6%).
Permanent discontinuation of TECVAYLI due to adverse reactions occurred in 1.2% of patients. Adverse reactions resulting in permanent discontinuation of TECVAYLI included pneumonia (adenoviral and pneumocystis jirovecii pneumonia in the same patient) and hypercalcemia.
Dosage interruptions of TECVAYLI due to an adverse reaction occurred in 73% of patients. Adverse reactions which required dosage interruption in >5% of patients included neutropenia, pneumonia, pyrexia, cytokine release syndrome, upper respiratory tract infection, and COVID-19.
The most common adverse reactions (≥20%) were pyrexia, CRS, musculoskeletal pain, injection site reaction, fatigue, upper respiratory tract infection, nausea, headache, pneumonia, and diarrhea. The most common Grade 3 to 4 laboratory abnormalities (≥20%) were decreased lymphocytes, decreased neutrophils, decreased white blood cells, decreased hemoglobin, and decreased platelets.
Table 10 summarizes the adverse reactions in MajesTEC-1.
Table 10: Adverse Reactions (≥10%) in Patients with Multiple Myeloma Who Received TECVAYLI in MajesTEC-1 Adverse Reactions TECVAYLI
(N=165)Any Grade
(%)Grade 3 or 4
(%)Adverse reactions were graded based on CTCAE Version 4.03, with the exception of CRS, which was graded per ASTCT 2019 criteria. - *
- Only grade 3 adverse reactions occurred.
- †
- Injection site reaction includes application site erythema, injection site bruising, injection site cellulitis, injection site discomfort, injection site erythema, injection site hematoma, injection site induration, injection site inflammation, injection site edema, injection site pruritus, injection site rash, injection site reaction and injection site swelling.
- ‡
- Fatigue includes asthenia and fatigue.
- §
- Pain includes ear pain, flank pain, groin pain, oropharyngeal pain, pain, pain in jaw, toothache and tumor pain.
- ¶
- Edema includes face edema, fluid overload, fluid retention, edema peripheral and peripheral swelling.
- #
- Hypogammaglobulinemia includes hypogammaglobulinemia and hypoglobulinemia.
- Þ
- Musculoskeletal pain includes arthralgia, back pain, muscle discomfort, musculoskeletal chest pain, musculoskeletal pain, myalgia, neck pain, non-cardiac chest pain and pain in extremity.
- ß
- Upper respiratory tract infection includes bronchitis, influenza like illness, nasopharyngitis, pharyngitis, respiratory tract infection, respiratory tract infection bacterial, rhinitis, rhinovirus infection, sinusitis, tracheitis, upper respiratory tract infection and viral upper respiratory tract infection.
- à
- Pneumonia includes COVID-19 pneumonia, enterobacter pneumonia, lower respiratory tract infection, metapneumovirus pneumonia, pneumocystis jirovecii pneumonia, pneumonia, pneumonia adenoviral, pneumonia klebsiella, pneumonia moraxella, pneumonia pneumococcal, pneumonia pseudomonal, pneumonia respiratory syncytial viral, pneumonia staphylococcal and pneumonia viral.
- è
- Includes the following fatal adverse reactions: hemorrhage (n=1), pneumonia (n=3).
- ð
- Urinary tract infection includes cystitis, cystitis escherichia, cystitis klebsiella, escherichia urinary tract infection, urinary tract infection and urinary tract infection bacterial.
- ø
- Motor dysfunction includes cogwheel rigidity, dysgraphia, dysphonia, gait disturbance, hypokinesia, muscle rigidity, muscle spasms, muscular weakness, peroneal nerve palsy, psychomotor hyperactivity, tremor and VI th nerve paralysis.
- ý
- Sensory neuropathy includes dysesthesia, hypoesthesia, hypoesthesia oral, neuralgia, paresthesia, paresthesia oral, peripheral sensory neuropathy, sciatica and vestibular neuronitis.
- £
- Encephalopathy includes agitation, apathy, aphasia, confusional state, delirium, depressed level of consciousness, disorientation, dyscalculia, hallucination, lethargy, memory impairment, mental status changes and somnolence.
- ¥
- Hemorrhage includes conjunctival hemorrhage, epistaxis, hematoma, hematuria, hemoperitoneum, hemorrhoidal hemorrhage, lower gastrointestinal hemorrhage, melena, mouth hemorrhage and subdural hematoma.
- Œ
- Hypertension includes essential hypertension and hypertension.
- œ
- Cough includes allergic cough, cough, productive cough and upper-airway cough syndrome.
- Ɖ
- Cardiac arrhythmia includes atrial flutter, cardiac arrest, sinus bradycardia, sinus tachycardia, supraventricular tachycardia, tachycardia and ventricular tachycardia.
- A
- Acute kidney injury includes acute kidney injury and renal impairment.
General disorders and administration site conditions Pyrexia 76 3 * Injection site reaction † 37 0.6 * Fatigue ‡ 33 2.4 * Chills 16 0 Pain § 15 1.8 * Edema ¶ 13 0 Immune system disorders Cytokine release syndrome 72 0.6 * Hypogammaglobulinemia # 11 1.2 * Musculoskeletal and connective tissue disorders Musculoskeletal pain Þ 44 4.2 * Bone pain 16 3 * Infections Upper respiratory tract infection ß 26 2.4 * Pneumonia àè 24 15 Urinary tract infection ð 11 5 * Gastrointestinal disorders Nausea 25 0.6 * Diarrhea 21 2.4 * Constipation 18 0 Vomiting 12 0.6 * Nervous system disorders Headache 25 0.6 * Motor dysfunction ø 16 0 Sensory neuropathy ý 15 1.2 * Encephalopathy £ 13 0 Vascular disorders Hypotension 18 1.2 * Hemorrhage ¥è 12 1.8 Hypertension Œ 12 4.8 * Respiratory, thoracic, and mediastinal disorders Hypoxia 18 1.8 Cough œ 15 0 Cardiac disorders Cardiac arrhythmia Ɖ 16 1.8 Metabolism and nutrition disorders Decreased appetite 11 0.6 * Renal and urinary disorders Acute kidney injury A 11 3.6 Clinically relevant adverse reactions in <10% of patients who received TECVAYLI included febrile neutropenia, sepsis, ICANS, seizure, Guillain-Barré syndrome, hepatic failure, and new onset or reactivated viral infections (including adenovirus, hepatitis B virus (HBV), cytomegalovirus (CMV), varicella zoster virus (VZV), herpes simplex virus (HSV), and progressive multifocal leukoencephalopathy (PML).
Table 11 summarizes laboratory abnormalities in MajesTEC-1.
Table 11: Select Laboratory Abnormalities (≥30%) That Worsened from Baseline in Patients with Multiple Myeloma Who Received TECVAYLI in MajesTEC-1 Laboratory Abnormality TECVAYLI
(N=165 *)All Grades (%) Grade 3 or 4 (%) Laboratory toxicity grades are derived based on the NCI CTCAE (National Cancer Institute Common Terminology Criteria for Adverse Events) Version 4.03. - *
- The denominator used to calculate the rate varied from 164 to 165 based on the number of patients with a baseline value and at least one post-treatment value.
Hematology Lymphocyte count decreased 92 84 White blood cell decreased 86 41 Neutrophil count decreased 84 56 Platelet count decreased 71 22 Hemoglobin decreased 67 33 Chemistry Albumin decreased 68 6 Alkaline phosphatase increased 42 2.4 Phosphorus decreased 38 13 Gamma-glutamyl transferase increased 37 8 Sodium decreased 35 10 Aspartate aminotransferase increased 34 1.2 Calcium (corrected) decreased 31 1.2 Creatinine increased 30 3 -
7 DRUG INTERACTIONS
TECVAYLI causes release of cytokines [see Clinical Pharmacology (12.2)] that may suppress activity of cytochrome P450 (CYP) enzymes, resulting in increased exposure of CYP substrates. The highest risk of drug-drug interaction is expected to occur from initiation of TECVAYLI step-up dosing schedule up to 7 days after the first treatment dose and during and after CRS [see Warnings and Precautions (5.1)] . Monitor for toxicity or concentrations of drugs that are CYP substrates where minimal concentration changes may lead to serious adverse reactions. Adjust the dose of the concomitant CYP substrate drug as needed.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on the mechanism of action, TECVAYLI may cause fetal harm when administered to a pregnant woman [see Clinical Pharmacology (12.1)] . There are no available data on the use of TECVAYLI in pregnant women to evaluate for a drug associated risk. No animal reproductive or developmental toxicity studies have been conducted with TECVAYLI. Teclistamab-cqyv causes T-cell activation and cytokine release; immune activation may compromise pregnancy maintenance. Human immunoglobulin G (IgG) is known to cross the placenta; therefore, teclistamab-cqyv has the potential to be transmitted from the mother to the developing fetus. Advise women of the potential risk to the fetus.
TECVAYLI is associated with hypogammaglobulinemia, therefore, assessment of immunoglobulin levels in newborns of mothers treated with TECVAYLI should be considered.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
8.2 Lactation
Risk Summary
There are no data on the presence of teclistamab-cqyv in human milk, the effect on the breastfed child, or the effects on milk production. Maternal IgG is known to be present in human milk. The effects of local gastrointestinal exposure and limited systemic exposure in the breastfed child to TECVAYLI are unknown. Because of the potential for serious adverse reactions in a breastfed child, advise women not to breastfeed during treatment with TECVAYLI and for 5 months after the last dose.
8.3 Females and Males of Reproductive Potential
TECVAYLI may cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)] .
8.4 Pediatric Use
The safety and efficacy of TECVAYLI have not been established in pediatric patients.
8.5 Geriatric Use
Of the 165 patients with relapsed or refractory multiple myeloma treated with TECVAYLI in MajesTEC-1 at the recommended dosage, 48% were 65 years of age or older, and 15% were 75 years of age or older. No overall differences in safety or effectiveness were observed between patients 65 to 74 years of age compared to younger patients. There is an insufficient number of patients 75 years of age or older to assess whether there are differences in safety or effectiveness.
-
11 DESCRIPTION
Teclistamab-cqyv, a bispecific B-cell maturation antigen (BCMA)-directed CD3 T-cell engager, is a humanized immunoglobulin G4-proline, alanine, alanine (IgG4-PAA) antibody. Teclistamab-cqyv is produced in Chinese Hamster Ovary (CHO) cells using recombinant DNA technology. Teclistamab-cqyv consists of an anti-BCMA heavy chain and light chain and an anti-CD3 heavy chain and light chain with two interchain disulfide bonds connecting the two arms. The molecular weight of teclistamab-cqyv is approximately 146 kDa.
TECVAYLI ®(teclistamab-cqyv) injection is a sterile, preservative-free, clear to slightly opalescent, colorless to light yellow solution supplied in a single-dose vial for subcutaneous administration.
Each TECVAYLI 3 mL single-dose vial contains 30 mg of teclistamab-cqyv, edetate disodium (0.054 mg), glacial acetic acid (0.72 mg), polysorbate 20 (1.2 mg), sodium acetate (2.7 mg), sucrose (240 mg), and Water for Injection, USP.
Each TECVAYLI 1.7 mL single-dose vial contains 153 mg of teclistamab-cqyv, edetate disodium (0.031 mg), glacial acetic acid (0.41 mg), polysorbate 20 (0.68 mg), sodium acetate (1.5 mg), sucrose (140 mg), and Water for Injection, USP.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Teclistamab-cqyv is a bispecific T-cell engaging antibody that binds to the CD3 receptor expressed on the surface of T-cells and B-cell maturation antigen (BCMA) expressed on the surface of multiple myeloma cells and some healthy B-lineage cells.
In vitro, teclistamab-cqyv activated T-cells, caused the release of various proinflammatory cytokines, and resulted in the lysis of multiple myeloma cells.
12.2 Pharmacodynamics
Serum concentrations of cytokines (IL-6, IL-10, TNF-α, and IFN-γ) and IL-2R were measured before and after administration of step-up dose 1, step-up dose 2, and the first three treatment doses of TECVAYLI. Increased concentrations of IL-6, IL-10, and IL-2R were observed during this period.
12.3 Pharmacokinetics
The C maxand AUC tauof teclistamab-cqyv after the first subcutaneous treatment dose increase proportionally over a dosage range of 0.08 mg/kg to 3 mg/kg (0.05 to 2 times the approved recommended treatment dosage). Ninety percent of steady state exposure was achieved after 12 weekly treatment doses. The mean accumulation ratio between the first and 13 thweekly treatment dose of teclistamab-cqyv 1.5 mg/kg was 4.2-fold for C max, 4.1-fold for C trough, and 5.3-fold for AUC tau.
The C max, C trough, and AUC tauof teclistamab-cqyv are presented in Table 12.
Table 12: Pharmacokinetic Parameters of Teclistamab-cqyv for the 13 thWeekly Treatment Dose of 1.5 mg/kg * Pharmacokinetic Parameter Teclistamab-cqyv
Geometric Mean (CV%)SD = standard deviation; C max= Maximum serum teclistamab-cqyv concentration; C trough= Serum teclistamab-cqyv concentration prior to next dose; CV = geometric coefficient of variation; AUC tau= Area under the concentration-time curve over the weekly dosing interval. - *
- Following administration of teclistamab-cqyv in patients with relapsed or refractory multiple myeloma (MajesTEC-1).
C max(mcg/mL) 23.8 (55%) C trough(mcg/mL) 21.1 (63%) AUC tau(mcg∙h/mL) 3,838 (57%) Absorption
The mean bioavailability of teclistamab-cqyv was 72% when administered subcutaneously. The median (range) T maxof teclistamab-cqyv after the first and 13 thweekly treatment doses were 139 (19 to 168) hours and 72 (24 to 168) hours, respectively.
Distribution
The mean (coefficient of variation [CV]%) volume of distribution of teclistamab-cqyv was 5.63 L (29%).
Elimination
Teclistamab-cqyv clearance decreases over time, with a mean (CV%) maximal reduction from baseline to the 13 thweekly treatment dose of 40.8% (56%). The geometric mean (CV%) clearance is 0.472 L/day (64%) at the 13 thweekly treatment dose. Patients who discontinue teclistamab-cqyv after the 13 thweekly treatment dose are expected to have a 50% reduction from C maxin teclistamab-cqyv concentration at a median (5 thto 95 thpercentile) time of 15 (7 to 33) days after T maxand a 97% reduction from C maxin teclistamab-cqyv concentration at a median time of 69 (32 to 163) days after T max.
Specific Populations
The volume of distribution and clearance of teclistamab-cqyv increase with increasing body weight (41 kg to 139 kg).
There were no clinically significant differences in the pharmacokinetics of teclistamab-cqyv based on age (24 to 84 years), sex, race (White, Black or African American), ethnicity (Hispanic/Latino, not Hispanic/Latino), mild or moderate renal impairment (estimated glomerular filtration rate [eGFR] by Modification of Diet in Renal Disease [MDRD] method: 30 to 89 mL/min), or mild hepatic impairment (total bilirubin less than or equal to upper limit of normal [ULN] with AST greater than ULN or total bilirubin greater than 1 to 1.5 times ULN with any AST). The effects of severe renal impairment (eGFR less than 30 mL/min) or moderate to severe hepatic impairment (total bilirubin greater than 1.5 times ULN with any AST) on the pharmacokinetics of teclistamab-cqyv are unknown.
12.6 Immunogenicity
The observed incidence of anti-drug antibodies is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of anti-drug antibodies in the studies described below with the incidence of anti-drug antibodies in other studies, including those of teclistamab-cqyv or of other teclistamab products.
During treatment in MajesTEC-1 (up to 27 months), 1/186 (0.5%) of patients treated with subcutaneous TECVAYLI at various dosages developed anti-teclistamab-cqyv antibodies. Because of the low occurrence of anti-drug antibodies, the effect of these antibodies on the pharmacokinetics, pharmacodynamics, safety, and/or effectiveness of teclistamab products is unknown.
- 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
The efficacy of TECVAYLI was evaluated in patients with relapsed or refractory multiple myeloma in a single-arm, open-label, multi-center study (MajesTEC-1, NCT03145181 [Phase 1] and NCT04557098 [Phase 2]). The study included patients who had previously received at least three prior therapies, including a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 monoclonal antibody. The study excluded patients who had stroke, seizure, allogeneic stem cell transplantation within the past 6 months, Eastern Cooperative Oncology Group (ECOG) performance score of 2 or higher, known active CNS involvement or clinical signs of meningeal involvement of multiple myeloma, or active or documented history of autoimmune disease, with the exception of vitiligo, Type 1 diabetes, and prior autoimmune thyroiditis.
Patients received step-up doses of 0.06 mg/kg and 0.3 mg/kg of TECVAYLI followed by TECVAYLI 1.5 mg/kg subcutaneously once weekly thereafter until disease progression or unacceptable toxicity [see Dosage and Administration (2.1)] .
The efficacy population included 110 patients. The median age was 66 (range: 33 to 82) years with 16% of patients 75 years of age or older; 56% were male; 91% were White, 5% were Black or African American, 3% were Asian. The International Staging System (ISS) at study entry was Stage I in 50%, Stage II in 38%, and Stage III in 12% of patients. High-risk cytogenetics (presence of del(17p), t(4;14) and t(14;16)) were present in 25% of patients. Seventeen percent of patients had extramedullary plasmacytomas. Patients with prior BCMA-targeted therapy were not included in the efficacy population.
The median number of prior lines of therapy was 5 (range: 2 to 14); 78% of patients had received at least 4 prior lines of therapy. Eighty-one percent of patients received prior stem cell transplantation. All patients had received prior therapy with a proteasome inhibitor, an immunomodulatory agent and an anti-CD38 monoclonal antibody, and 76% were triple-class refractory (refractory to a proteasome inhibitor, an immunomodulatory agent and an anti-CD38 monoclonal antibody).
Efficacy was established based on overall response rate (ORR) as determined by the Independent Review Committee (IRC) assessment using International Myeloma Working Group (IMWG) 2016 criteria (see Table 13).
The median time to first response was 1.2 months (range: 0.2 to 5.5 months). With a median follow-up of 7.4 months among responders, the estimated duration of response (DOR) rate was 90.6% (95% CI: 80.3%, 95.7%) at 6 months and 66.5% (95% CI: 38.8%, 83.9%) at 9 months.
Table 13: Efficacy Results for MajesTEC-1 N=110 NE=not estimable - *
- Complete response or better = Stringent complete response (sCR) + complete response (CR).
Overall response rate (ORR: sCR+CR+VGPR+PR) n(%) 68 (61.8) 95% CI (%) (52.1, 70.9) Complete response (CR) or better * 31 (28.2) Very good partial response (VGPR) 32 (29.1) Partial response (PR) 5 (4.5) Duration of Response (DOR) (months) DOR (Months): Median (95% CI) NE (9.0, NE) -
16 HOW SUPPLIED/STORAGE AND HANDLING
TECVAYLI ®(teclistamab-cqyv) injection is a sterile, preservative-free, clear to slightly opalescent, colorless to light yellow solution supplied as follows:
- One 30 mg/3 mL (10 mg/mL) single-dose vial in a carton: NDC: 57894-449-01
- One 153 mg/1.7 mL (90 mg/mL) single-dose vial in a carton: NDC: 57894-450-01
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Cytokine Release Syndrome (CRS)
Discuss the signs and symptoms associated with CRS, including fever, hypoxia, chills, hypotension, sinus tachycardia, headache, and elevated liver enzymes. Advise patients to immediately contact their healthcare provider if they experience any signs or symptoms of CRS. Advise patients that they will be hospitalized for 48 hours after administration of all doses within the TECVAYLI step-up dosing schedule [see Dosage and Administration (2.4)and Warnings and Precautions (5.1)].
Neurologic Toxicity including ICANS
Discuss the signs and symptoms associated with neurologic toxicity, including ICANS, including headache, confusion, dysgraphia, motor dysfunction, neuropathy, or encephalopathy. Advise patients to immediately contact their healthcare provider if they experience any signs or symptoms of neurologic toxicity. Advise patients to refrain from driving or operating heavy or potentially dangerous machinery during and for 48 hours after completion of TECVAYLI step-up dosing schedule and in the event of new onset of any neurologic toxicity symptoms until neurologic toxicity resolves [see Dosage and Administration (2.4)and Warnings and Precautions (5.2)].
TECVAYLI and TALVEY REMS
TECVAYLI is available only through a restricted program called TECVAYLI and TALVEY REMS. Inform patients that they will be given a Patient Wallet Card that they should carry with them at all times and show to all of their healthcare providers. This card describes signs and symptoms of CRS and neurologic toxicity which, if experienced, should prompt the patient to immediately seek medical attention [see Warnings and Precautions (5.3)].
Hepatotoxicity
Advise patients that liver enzyme elevations may occur and that they should report symptoms that may indicate liver toxicity, including fatigue, anorexia, right upper abdominal discomfort, dark urine, or jaundice [see Warnings and Precautions (5.4)].
Infections
Discuss the signs and symptoms of infection [see Dosage and Administration (2.4)and Warnings and Precautions (5.5)] .
Neutropenia
Discuss the signs and symptoms associated with neutropenia and febrile neutropenia [see Dosage and Administration (2.4)and Warnings and Precautions (5.6)] .
Hypersensitivity and Other Administration Reactions
Advise patients to immediately seek medical attention for any signs and symptoms of systemic administration-related reactions. Advise patients that local injection-site reactions may occur and to report any severe reactions [see Warnings and Precautions (5.7)].
Embryo-Fetal Toxicity
Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females of reproductive potential to inform their healthcare provider if they are pregnant or become pregnant. Advise females of reproductive potential to use effective contraception during treatment with TECVAYLI and for 5 months after the last dose [see Warnings and Precautions (5.8)and Use in Specific Populations (8.1, 8.3)] .
Lactation
Advise women not to breastfeed during treatment with TECVAYLI and for 5 months after the last dose [see Use in Specific Populations (8.2)] .
- SPL UNCLASSIFIED SECTION
-
MEDICATION GUIDE
MEDICATION GUIDE
TECVAYLI ®[tek vay' lee]
(teclistamab-cqyv) injection, for subcutaneous useThis Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: Aug 2025 What is the most important information I should know about TECVAYLI?
TECVAYLI may cause side effects that are serious, life-threatening or lead to death, including Cytokine Release Syndrome (CRS) and Neurologic problems.
Call your healthcare provider right away if you develop any of the signs or symptoms of CRS or neurologic problems listed below at any time during your treatment with TECVAYLI:
Cytokine Release Syndrome (CRS). Signs and symptoms of CRS may include:- fever (100.4 °F or higher)
- difficulty breathing
- chills
- dizziness or lightheadedness
- fast heartbeat
- feeling anxious
- confusion or restlessness
- headache
- increased liver enzymes in your blood
Neurologic problems. Symptoms of neurologic problems with TECVAYLI include: - headache
- jerking movements
- rigid muscles
- feeling restless
- numbness and tingling (feeling like "pins and needles")
- confusion
- trouble speaking
- muscle spasms
- tremor
- double vision
- changes in your handwriting
- problems walking
- muscle weakness in your body or face
- hearing loss
- burning, throbbing, or stabbing pain
- Due to the risk of CRS and neurologic symptoms, you should be hospitalized for 48 hours after all doses of TECVAYLI that are part of the "step-up dosing schedule." The "step-up dosing schedule" is when you receive the first 2 doses of TECVAYLI, which are called "step-up" doses, and then you receive the first "treatment dose" of TECVAYLI. After "step-up" dose 1 of TECVAYLI, the dose of TECVAYLI is increased. After "step-up" dose 2, the dose is increased again when you receive the first "treatment dose" of TECVAYLI.
- "Step-up dose 1" is given on day 1 of treatment. "Step-up dose 2" is usually given on day 4 of treatment. The first "treatment dose" is usually given on day 7 of treatment.
- Your healthcare provider will decide when you will receive "step-up dose 2" and your first "treatment dose."
- "Step-up" dose 2 may be given between 2 to 4 days after "step-up" dose 1, or up to 7 days after "step-up" dose 1 if you have certain side effects with TECVAYLI.
- Your first "treatment dose" may be given between 2 to 4 days after "step-up" dose 2, or up to 7 days after "step-up" dose 2 if you have certain side effects with TECVAYLI.
- Your healthcare provider will decide the number of days to wait between your doses of TECVAYLI as well as how many treatments you will receive.
- If your dose of TECVAYLI is delayed for any reason, you may need to repeat the "step-up dosing schedule" to receive TECVAYLI.
- Before each "step-up" dose and your first "treatment dose" of TECVAYLI you will receive medicines to help reduce your risk of CRS. Your healthcare provider will decide if you need to receive medicines to help reduce your risk of CRS with future doses.
- Your healthcare provider will monitor you for signs and symptoms of CRS and neurologic problems during treatment with TECVAYLI, as well as other side effects and treat you as needed.
- Do not drive or operate heavy or dangerous machinery during and for 48 hours after your TECVAYLI "step-up dosing schedule" is completed, or at any time during treatment with TECVAYLI if you develop new neurologic symptoms until the symptoms go away.
You will receive a Patient Wallet Card from your healthcare provider. Carry the Patient Wallet Card with you at all times and show it to all of your healthcare providers.The Patient Wallet Card lists signs and symptoms of CRS and neurologic problems.
Get medical help right away if you develop any of the signs and symptoms listed on the Patient Wallet Card.You may need to be treated in a hospital.- If you have any questions about TECVAYLI, ask your healthcare provider.
- Your healthcare provider may temporarily stop or completely stop your treatment with TECVAYLI if you develop CRS, neurologic problems or any other side effects that are severe.
What is TECVAYLI?
TECVAYLI is a prescription medicine to treat adults with multiple myeloma who:- have already received at least 4 treatment regimens, including a proteasome inhibitor, an immunomodulatory agent and an anti-CD38 monoclonal antibody to treat their multiple myeloma, and
- their cancer has come back or did not respond to prior treatment
Before you receive TECVAYLI, tell your healthcare provider about all of your medical conditions, including if you: - have an infection
- are pregnant or plan to become pregnant. TECVAYLI may harm your unborn baby.
- Your healthcare provider should do a pregnancy test before you start treatment with TECVAYLI.
- You should use effective birth control (contraception) during treatment and for 5 months after your last dose of TECVAYLI.
- Tell your healthcare provider right away if you become pregnant or think that you may be pregnant during treatment with TECVAYLI.
- are breastfeeding or plan to breastfeed. It is not known if TECVAYLI passes into your breast milk. Do not breastfeed during treatment and for 5 months after your last dose of TECVAYLI.
How will I receive TECVAYLI? - TECVAYLI will be given to you by your healthcare provider as an injection under your skin (subcutaneous injection), usually in your stomach-area (abdomen), your thigh or another area of your body may be injected.
- See " What is the most important information I should know" at the beginning of this Medication Guide for information about how you will receive TECVAYLI.
- If you miss any appointments, call your healthcare provider as soon as possible to reschedule your appointment. It is important for you to be monitored closely for side effects during treatment with TECVAYLI.
What are the possible side effects of TECVAYLI?
TECVAYLI may cause serious side effects, including:- See " What is the most important information I should know about TECVAYLI?"
- Liver problems. TECVAYLI can cause liver problems that may lead to death. Increased bilirubin and liver enzymes in your blood are common with TECVAYLI and can also sometimes be severe These increases in liver enzymes can happen with or without you also having CRS. Your healthcare provider will monitor you for these problems before you start and during treatment with TECVAYLI. Tell your healthcare provider if you develop any symptoms of a liver problem including:
- tiredness
- loss of appetite
- pain in your right upper stomach-area (abdomen)
- dark urine
- yellowing of your skin or white part of your eyes
-
Infections.Upper respiratory tract infections and pneumonia are common with TECVAYLI. TECVAYLI can cause bacterial and viral infections that are severe, life-threatening, or that may lead to death.
- Your healthcare provider will monitor you for signs and symptoms of infection before and during treatment with TECVAYLI.
- Your healthcare provider may prescribe medicines for you to help prevent infections and treat you as needed if you develop an infection during treatment with TECVAYLI.
- Tell your healthcare provider right away if you get a fever, chills or any signs or symptoms of an infection.
- Decreased white blood cell counts.Decreased white blood cell counts are common with TECVAYLI and can also be severe. Fever sometimes also happens with low white blood cell counts and may be a sign that you have an infection. Your healthcare provider will check your blood cell counts before you start and during treatment with TECVAYLI, and treat you as needed.
-
Allergic reactions and injection site reactions.TECVAYLI can cause allergic reactions that can affect your whole body (systemic), and also cause injection site reactions.
- Some people taking TECVAYLI can develop symptoms of an allergic reaction that can affect your whole body and may include fever or a swollen tongue. Get medical help right away if you develop symptoms of an allergic reaction during treatment with TECVAYLI.
- Injection site reactions are common with TECVAYLI and can include: redness, heat, swelling, bruising, bacterial skin infection (cellulitis), discomfort, blood collection under the skin at the injection site (hematoma), and rash. Tell your healthcare provider if you develop any severe injection site reactions.
The most common side effects of TECVAYLI include:- fever
- pain in your joints and muscles, back and chest muscles, and in your arms and legs
- tiredness and weakness
- upper respiratory tract infections and pneumonia. See " Infections" above.
- nausea
- headache
- diarrhea
The most common severe abnormal lab test results with TECVAYLI include: decreased white blood cells, red blood cells and platelets.
These are not all the possible side effects of TECVAYLI.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.General Information about TECVAYLI.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide.
You can ask your healthcare provider for information about TECVAYLI that is written for health professionals.What are the ingredients of TECVAYLI?
Active ingredient: teclistamab-cqyv
Inactive ingredients: edetate disodium, glacial acetic acid, polysorbate 20, sodium acetate, sucrose, Water for Injection
Manufactured by: Janssen Biotech, Inc., Horsham, PA 19044, USA
U.S. License Number 1864
For patent information: www.janssenpatents.com
© Johnson & Johnson and its affiliates 2022
For more information about TECVAYLI go to www.TECVAYLI.com or call 1-800-526-7736. -
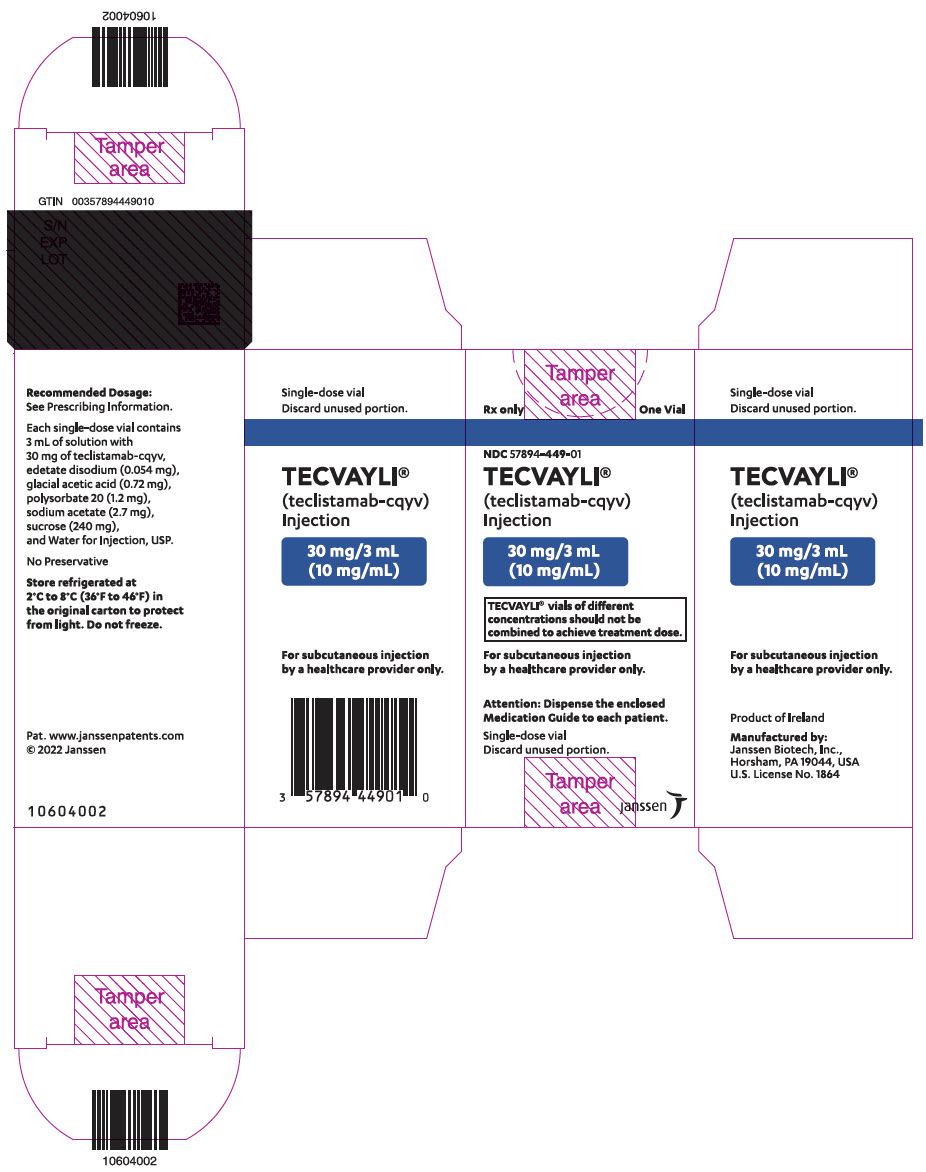
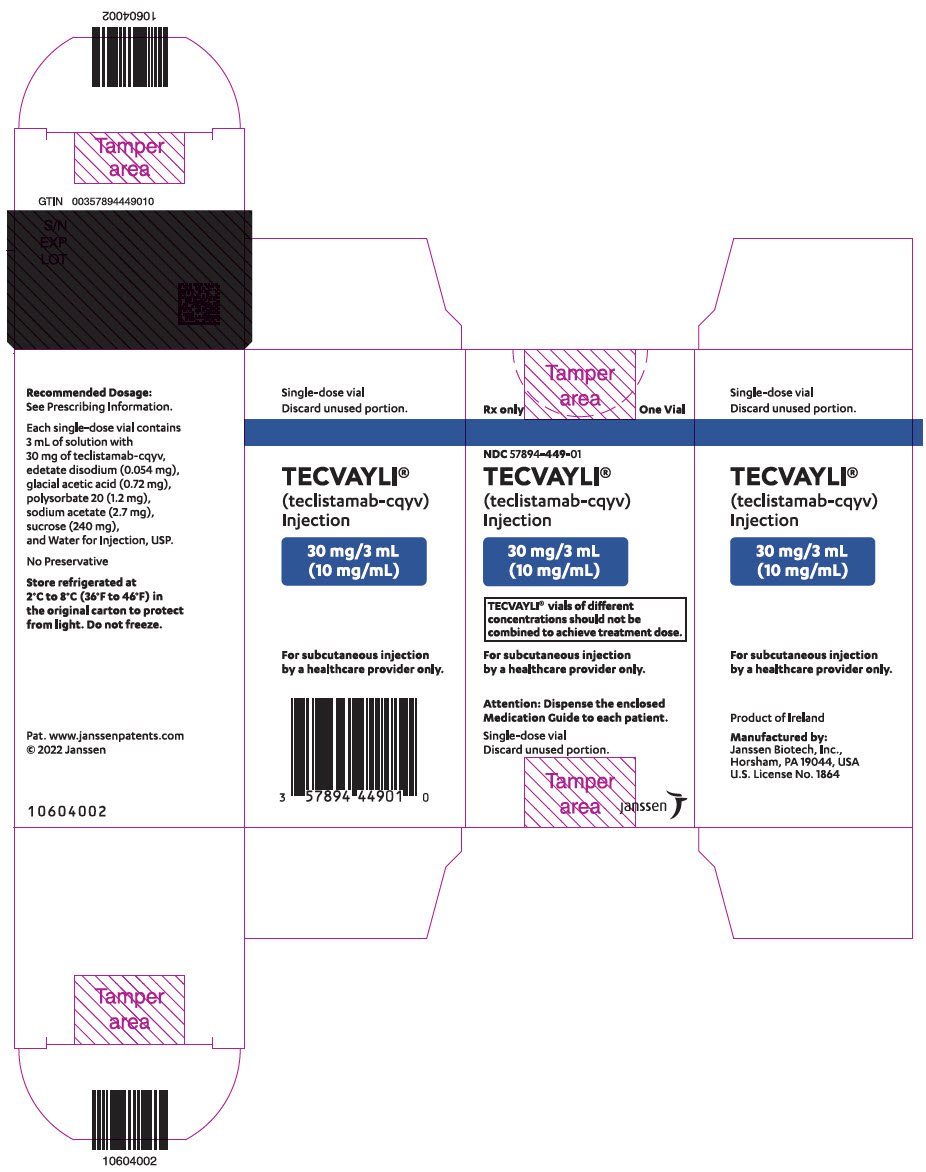
PRINCIPAL DISPLAY PANEL - 30 mg/3 mL Vial Carton
Rx only
One VialNDC 57894-449-01
TECVAYLI ®
(teclistamab-cqyv)
Injection30 mg/3 mL
(10 mg/mL)TECVAYLI ®vials of different
concentrations should not be
combined to achieve treatment dose.For subcutaneous injection
by a healthcare provider only.Attention: Dispense the enclosed
Medication Guide to each patient.Single-dose vial
Discard unused portion.janssen
-
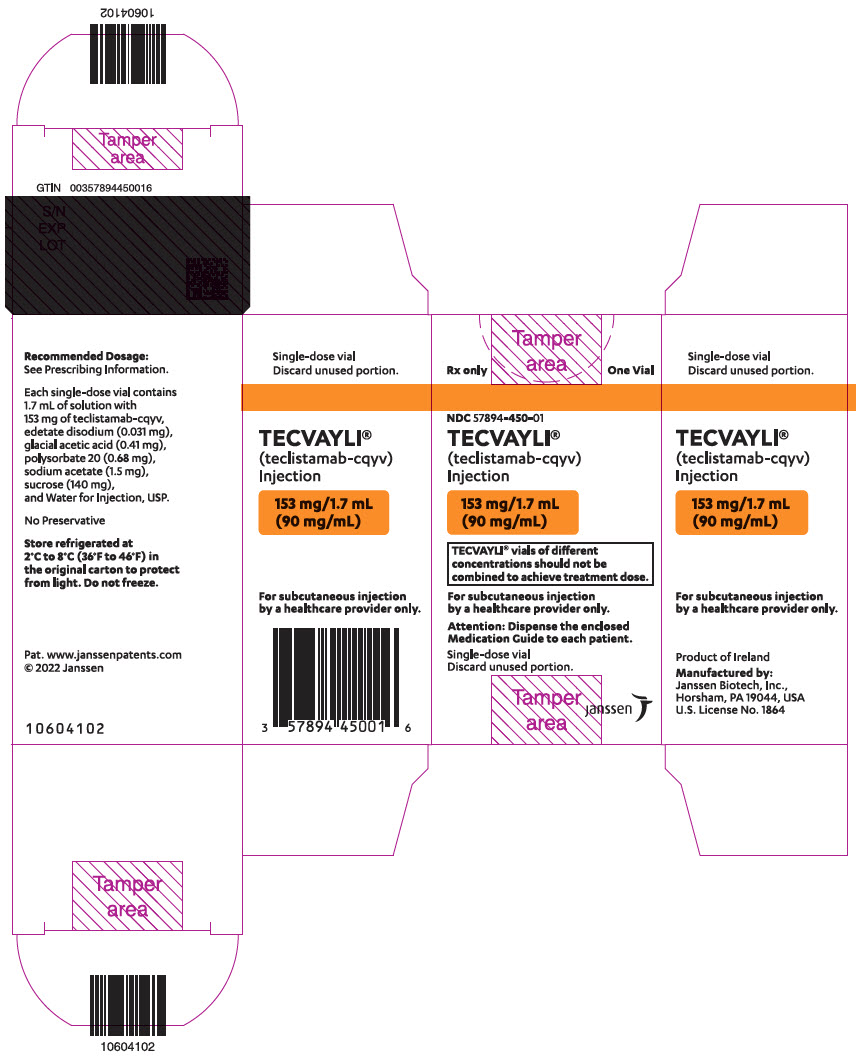
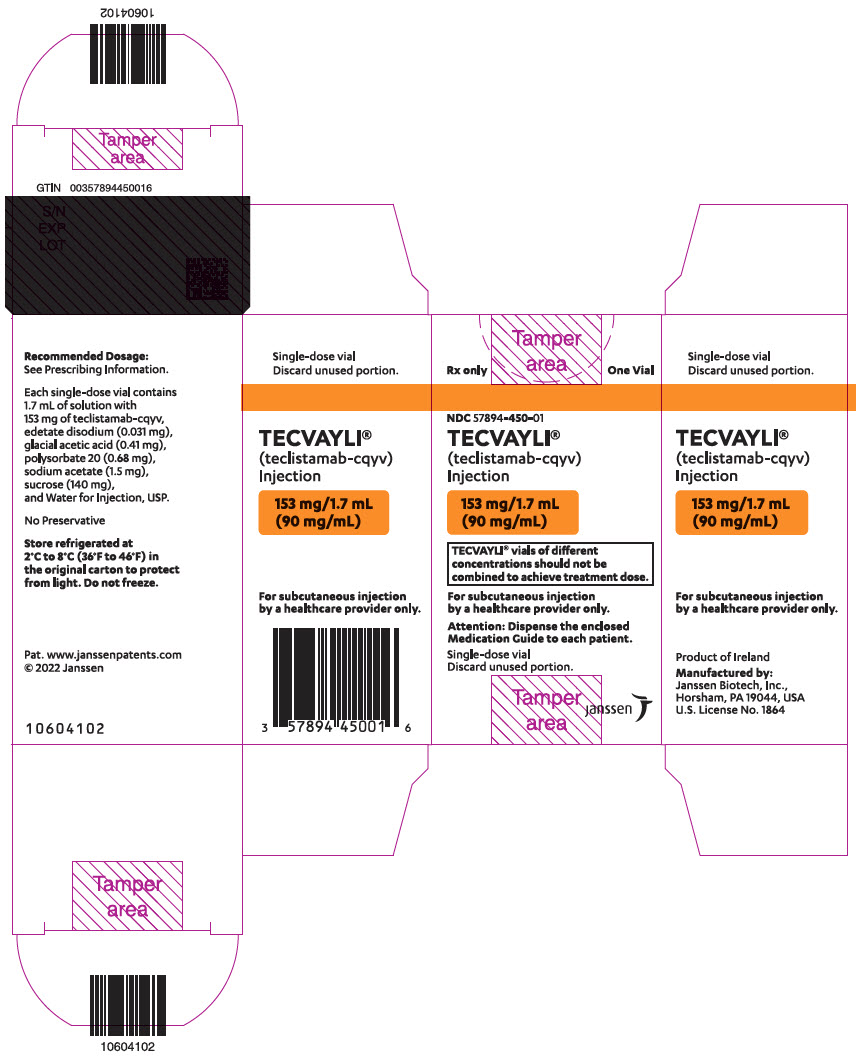
PRINCIPAL DISPLAY PANEL - 153 mg/1.7 mL Vial Carton
Rx only
One VialNDC 57894-450-01
TECVAYLI ®
(teclistamab-cqyv)
Injection153 mg/1.7 mL
(90 mg/mL)TECVAYLI ®vials of different
concentrations should not be
combined to achieve treatment dose.For subcutaneous injection
by a healthcare provider only.Attention: Dispense the enclosed
Medication Guide to each patient.Single-dose vial
Discard unused portion.janssen
-
INGREDIENTS AND APPEARANCE
TECVAYLI
teclistamab injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:57894-449 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TECLISTAMAB (UNII: 54534MX6Z9) (TECLISTAMAB - UNII:54534MX6Z9) TECLISTAMAB 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM ACETATE (UNII: 4550K0SC9B) ACETIC ACID (UNII: Q40Q9N063P) SUCROSE (UNII: C151H8M554) POLYSORBATE 20 (UNII: 7T1F30V5YH) EDETATE DISODIUM (UNII: 7FLD91C86K) WATER (UNII: 059QF0KO0R) Product Characteristics Color yellow (Colorless to light yellow) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:57894-449-01 1 in 1 CARTON 10/25/2022 1 3 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761291 10/25/2022 TECVAYLI
teclistamab injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:57894-450 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TECLISTAMAB (UNII: 54534MX6Z9) (TECLISTAMAB - UNII:54534MX6Z9) TECLISTAMAB 90 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM ACETATE (UNII: 4550K0SC9B) ACETIC ACID (UNII: Q40Q9N063P) SUCROSE (UNII: C151H8M554) POLYSORBATE 20 (UNII: 7T1F30V5YH) EDETATE DISODIUM (UNII: 7FLD91C86K) WATER (UNII: 059QF0KO0R) Product Characteristics Color yellow (Colorless to light yellow) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:57894-450-01 1 in 1 CARTON 10/25/2022 1 1.7 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761291 10/25/2022 Labeler - Janssen Biotech, Inc. (099091753) Establishment Name Address ID/FEI Business Operations Janssen Biotech, Inc. 038978363 api manufacture(57894-449, 57894-450) Establishment Name Address ID/FEI Business Operations AndersonBrecon, Inc. 053217022 pack(57894-449, 57894-450) , label(57894-449, 57894-450) Establishment Name Address ID/FEI Business Operations Patheon Manufacturing Services LLC 079415560 analysis(57894-449, 57894-450) , manufacture(57894-449, 57894-450) , label(57894-449, 57894-450) , pack(57894-449, 57894-450) Establishment Name Address ID/FEI Business Operations BioReliance Corporation 147227730 analysis(57894-449, 57894-450) Establishment Name Address ID/FEI Business Operations Janssen Biologics B.V. 409612918 api manufacture(57894-450, 57894-449) , analysis(57894-449, 57894-450) Establishment Name Address ID/FEI Business Operations Cilag AG 483237103 analysis(57894-449, 57894-450) Establishment Name Address ID/FEI Business Operations Biogen MA, Inc. 841087823 api manufacture(57894-449, 57894-450) , analysis(57894-449, 57894-450) Establishment Name Address ID/FEI Business Operations Janssen Sciences Ireland UC 986030167 api manufacture(57894-450, 57894-449) , analysis(57894-449, 57894-450)