Label: PROIN ER- phenylpropanolamine hydrochloride tablet, extended release
- NDC Code(s): 49427-346-44, 49427-346-57
- Packager: Pegasus Laboratories, Inc.
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Animal Drug Application
Drug Label Information
Updated December 20, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- GENERAL PRECAUTIONS
-
DESCRIPTION:
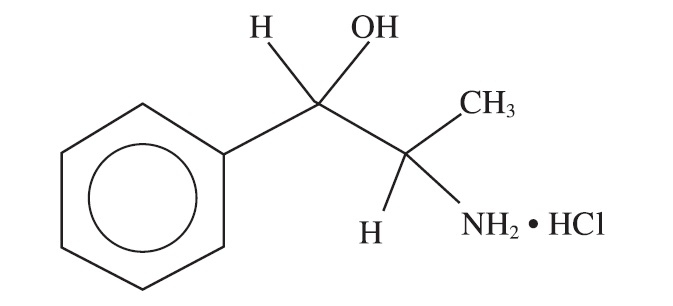
Description: PROIN ER (phenylpropanolamine hydrochloride extended-release tablets) is a sympathomimetic amine closely related to ephedrine. Phenylpropanolamine hydrochloride (PPA) is the nonproprietary designation for benzenemethanol, a-(1-aminoethyl)-hydrochloride, (R*, S*)-, (±). The empirical formula is C9H13NO•HCl and the molecular weight is 187.67. It is a white crystalline compound having a slight aromatic odor. PPA is freely soluble in water and alcohol but is practically insoluble in ether, benzene and chloroform. The chemical structure of phenylpropanolamine hydrochloride is:
- INDICATION:
-
DOSAGE AND ADMINISTRATION:
The recommended dosage is 2 to 4 mg/kg (0.9 to 1.8 mg/lb) of body weight once daily according to Table 1 below. Administer PROIN ER with food (see Clinical Pharmacology). Do not split or crush tablets.
Dogs weighing less than 10 pounds cannot be safely dosed because tablet administration would result in a dose over 4 mg/kg.Table 1. Dose Administrationa
Body weight in pounds PROIN ER 10-20 18 mg 21-40 38 mg 41-80 74 mg 81-125b 145 mg a Body weight should be rounded to the nearest pound.
b Dogs exceeding 125 lbs should receive the appropriate combination of tablets to achieve the recommended dosage.
Dogs may transition from PROIN® Chewable Tablets to PROIN ER without a break in administration. However, do not alternate PROIN
ER with PROIN Chewable Tablets because the effectiveness and safety of interchangeable use have not been evaluated. - WARNINGS:
-
PRECAUTIONS:
Proin ER may mask signs of incontinence due to urinary tract infection. PROIN ER is not effective in dogs with incontinence due to neurologic disease or malformations.
PROIN ER may cause hypertension; therefore, use with caution in dogs with pre-existing heart disease, hypertension, liver disease,
kidney insufficiency, diabetes, glaucoma, and conditions with a predilection for hypertension.
Use with caution in dogs receiving sympathomimetic drugs, tricyclic antidepressants, or monoamine oxidase inhibitors as increased toxicity
may result. Use with caution in dogs administered halogenated gaseous anesthetics as this may increase the risk of cardiac arrhythmias.A laboratory study on human blood revealed that phenylpropanolamine (PPA) used in conjunction with aspirin may potentiate decreased platelet aggregation.1
PROIN ER may cause increased thirst; therefore, provide dogs with ample fresh water.
The safe use of PROIN ER has not been evaluated in dogs that are intended for breeding, or that are pregnant or lactating. -
ADVERSE REACTIONS:
Adverse Reactions are listed below for both PROIN ER (NADA Number 141-517) and PROIN Chewable Tablets (NADA 141-324).
PROIN ER (NADA 141-517)
In the open-label clinical study involving 119 dogs administered PROIN ER once a day for 180 days, the following adverse reactions were observed.Table 2. Number and percentage of dogs with adverse reactions in the 180-day open-label clinical study for PROIN ER
Adverse Reactions Total N = 119 Emesis 39 (32.8%) Body weight loss (≥5%) 34 (28.6%) Hypertension (≥160 mmHg) developed during studya 15 (12.6%) Diarrhea 20 (16.8%) Proteinuria 16 (13.4%) Tachycardia (≥160 bpm) 11 (9.2%) Lethargy 11 (9.2%) Decreased appetite 10 (8.4%) Urinary Tract Infection 10 (8.4%) Elevated Alkaline phosphatase and/or Alanine Aminotransferase 7 (6.0%) Hypoglycemia 4 (3.3%) Hypercalcemia 3 (2.5%) Increased BUN 2 (1.7%) Bradycardia (<60 bpm) 2 (1.7%) Seizures/twitching 2 (1.7%) a There were an additional 21 dogs enrolled with hypertension who remained hypertensive throughout the study.
During the first week of administration of PROIN ER, 15% of dogs had reported emesis, diarrhea, or decreased appetite which improved or resolved prior to the Day 21 visit.
Four deaths occurred during the study. One dog was euthanized for pulmonary metastasis and one dog for poor quality of life due to hindlimb weakness. One dog had emesis and died at home; upon necropsy a foreign body was present in the small intestine. The fourth dog had been treated for a urinary tract infection three weeks prior to sudden death of undetermined cause.
PROIN Chewable Tablets (NADA 141-324):Table 3 below includes the most common adverse reactions observed in the masked, placebo-controlled 28-day clinical study involving 123 PROIN Chewable Tablet-treated dogs and 61 placebo-treated dogs. In addition, one dog exhibited disorientation, nervousness, a 7.7% loss of body weight, and hypertension with proteinuria. A second dog exhibited restless behavior, lethargy, a 2.8% body weight loss, and proteinuria.
Table 3. Number and percentage of dogs with adverse reactions in the 28-day placebo-controlled clinical study for PROIN Chewable Tablets
Adverse Reactions PROIN Treated (N=123) Placebo Treated (N=61) Emesis 20.3% 8.2% Hypertension (≥ 160mm Hg)a 19.5% 14.7% Anorexia 16.3% 3.3% Body weight loss (> 5%) b 16.1% 6.8% Proteinuria 13.0% 8.2% Anxiety/aggression/behavior change 9.7% 3.2% Diarrhea 7.3% 9.8% Polydipsia 6.5% 9.8% Lethargy 5.7% 1.6% Musculoskeletal Disorder 3.2% 1.6% Insomnia/Sleep Disorder 2.5% 0.0% a One or more systolic blood pressure readings of ≥160mmHg.
b The "N" for weight loss is PROIN-treated N=118 and placebo N=59 because seven dogs did not have a final weight at the time of withdrawal from the study.
-
ADVERSE REACTIONS
One-hundred fifty-seven dogs continued into the 6-month open-label clinical study for PROIN Chewable Tablets. The most common adverse reactions are listed in Table 4 below. In addition, one dog exhibited progressively worsening hypertension with proteinuria. Five dogs enrolled in the study with pre-existing heart disease. Of these, one dog developed systolic failure with an unknown relation to treatment.
-
ADVERSE REACTIONS
Table 4: Number and percentage of dogs with adverse reactions in the 6-month open-label clinical study for PROIN Chewable Tablets
Adverse Reactions Total N= 125 Hypertension (≥ 160 mmHg)a 34.6% Body Weight Loss (> 5%) 24.8% Emesis 19.7% Proteinuria 15.3% Anorexia 10.2% Diarrhea 6.4% Lethargy 5.7% Anxiety/ behavior change/ aggression 5.7% a Percent of dogs with systolic blood pressures of ≥ 160 mmHg on day -7 were 30.2% and on day 0 were 33.3%.
-
ADVERSE REACTIONS
Post Approval Experience for PROIN Chewable Tablets (2015):
The following adverse reactions are based on voluntary, post approval reporting for PROIN Chewable Tablets (2015). Not all adverse events are reported to FDA/CVM. It is not always possible to reliably estimate the adverse event frequency or establish a causal relationship to product exposure using these data. The signs reported are listed in decreasing order of reporting frequency by body system:
Gastrointestinal: Emesis, anorexia, diarrhea, hypersalivation
Behavioral: Agitation, lethargy, vocalization, confusion
General body system: Polydipsia, weight loss, weakness, fever
Respiratory: Panting
Dermatological: Erythema, piloerection
Hepatic: Elevated serum alanine aminotransferase (ALT), elevated serum alkaline phosphatase (ALP)
Neurologic: Ataxia, seizures, tremors
Renal/Urinary: Renal failure, hematuria, urinary retention
Cardiovascular: Tachycardia, hypertension, bradycardia, arrhythmias
Sensory: Ophthalmic disorders, mydriasis and eye redness
In some cases, death, including euthanasia, has been reported. Sudden death was sometimes preceded by neurologic signs, vocalization,
or collapse. A necropsy of one dog revealed subarachnoidal and intraventricular hemorrhage in the brain.The following signs have been reported more often with a dose higher than the recommended dosage: agitation, arrhythmia, bradycardia, erythema, fever, hypersalivation, hypertension, lethargy, mydriasis, panting, piloerection, tachycardia, tremor, and urinary retention.
For a copy of the Safety Data Sheet (SDS) or to report suspected adverse drug events, contact Pegasus Laboratories at 1-800-874-9764. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or www.fda.gov/reportanimalae
-
INFORMATION FOR DOG OWNERS:
Always follow the dosage instructions for PROIN ER provided by your veterinarian. Give PROIN ER with food and do not split or crush the tablet. Monitor your dog after giving PROIN ER to be sure all of it was consumed. If you have difficulty giving PROIN ER, contact your veterinarian.
PROIN ER may cause increased thirst; therefore, provide dogs with ample fresh water.
If you forget to give your dog a dose, then resume dosing at the next scheduled dose. Keep PROIN ER in a secured location out of reach of dogs, cats, and other animals to prevent accidental ingestion or overdose.Contact your veterinarian immediately if the dog ingests more tablets than prescribed or if other pets ingest PROIN ER. In the case of accidental ingestion by humans, contact a physician immediately.
Contact your veterinarian if you notice restlessness, irritability, loss of appetite, the incontinence persists or worsens, or any other unusual signs.
Consult your veterinarian before administering PROIN ER with any other medications. -
CLINICAL PHARMACOLOGY:
Phenylpropanolamine is a chemical analogue of the endogenous sympathomimetic amines. It is an alpha-adrenergic agent which has been reported to increase urethral tone in dogs. 2 Its mechanism of action is not well determined, but it is believed to cause the release of norepinephrine by indirectly stimulating both the alpha and beta-adrenergic receptors of the smooth muscle to increase smooth muscle tone of the urethra, bladder neck, and the internal urethral sphincter. 3, 4
In a crossover pharmacokinetic study of PROIN ER in fed and fasted dogs, post-prandial drug administration was associated with approximately a 23% increase in the maximum plasma concentration (Cmax), but the area under the concentration vs time curve to the last
quantifiable concentration (AUClast) was similar in both fed and fasted states. The small decrease in the post-prandial AUClast appeared to
be attributable to the corresponding increase in the terminal elimination rate constant under the fed conditions. The time to Cmax (Tmax)
was more variable in the fasted state, ranging from 1.5 to 8 hours compared to 2 to 6 hours for the fed state. The elimination half-life (t ½) was also more variable in the fasted state, ranging from 3.89 to 10.35 hours compared to 2.98 to 7.81 hours for the fed state. -
EFFECTIVENESS:
Effectiveness of PROIN ER was demonstrated in a multi-center, prospective, open-label, 6-month study in client-owned dogs of various breeds. In this study, 119 dogs (113 spayed females and 6 neutered males, aged 1-16 years and weighing 4.9-81.8 kg) who were considered well controlled for signs of urinary incontinence (UI) while receiving PROIN Chewable Tablets for at least 30 days prior to study start were enrolled in the study. Of these dogs, 104 were evaluated for effectiveness. The owners continued to administer PROIN Chewable Tablets twice a day and recorded episodes of UI during a baseline period (Day -7 through Day -1). After the baseline period, the owners transitioned to administration of PROIN ER once a day, at the labeled dose (see Dosage and Administration), and recorded urinary accidents for 28 days.
The primary variable was the ratio of average daily incidence of UI during the 7 days preceding the Day 28 clinic visit compared to the baseline period. It was concluded that PROIN ER was effective for the control of urinary incontinence due to urethral sphincter hypotonus in dogs.Table 5: Clinical Effectiveness Results for PROIN ER
Ratio Number of Dogs N = 104 Ratio >1, indicating response measurement period was better than baseline period 19 (18.3%) Ratio of 1, indicating no difference between response measurement period and baseline period 75 (72.1%) Ratio <1, indicating response measurement period was worse than baseline period 10 (9.6%) The secondary outcome variable was owner assessment of the control of UI at the end of the 28 day study period. The owner assessment was “improved” for 13 (12.5%) dogs, “stayed the same” for 90 (86.5%) dogs and “worsened” for 1 dog (1%).
-
ANIMAL SAFETY:
The safety of PROIN ER was established based on the safety data from PROIN Chewable Tablets (see below) and a comparative analysis of pharmacokinetic (PK) data for PROIN ER and PROIN Chewable Tablets. The statistical analysis of observed and simulated post-prandial pharmacokinetic data resulted in confidence limits consistent with equal or lower oral bioavailability for PROIN ER when administered once daily versus PROIN Chewable Tablets when administered twice daily. Therefore, the safety data from PROIN Chewable Tablets could be applied to PROIN ER. Emesis and hyperemia of the ventral abdomen were observed during the PK studies.
Target Animal Safety Study (PROIN Chewable Tablets, NADA 141-324)
In a target animal safety study, PROIN was administered to 32 healthy male and female Beagle dogs at 0, 2, 6 and 10 mg/kg of body weight (0, 1, 3, and 5 times the recommended dose; 8 dogs per group) twice daily for 26 consecutive weeks. The most pronounced finding was a dose-dependent increase in blood pressure. Mean systolic blood pressure was increased in all PPA-treated groups compared to the control, but mean values for all 4 groups were within the normal range. Mean diastolic and mean MAP (mean arterial pressure) were higher in the 3X and 5X groups, and in the 1X males. Dogs in the 3X and 5X groups had more individual systolic, diastolic, and MAP values above the normal range than the control group dogs. A dose-dependent decrease in heart rate was observed in the 3X and 5X dogs. In the 0, 1, 3, and 5X groups, 5%, 34%, 44% and 40% of the total number of heart rates obtained from electrocardiograms for each group over the course of the study were below the normal range (70-120 beats per minute), with the lowest value being 51 bpm in 4 of the 1X group dogs. One dog in each of the 1X and 5X had an elevated heart rate between 150-180 beats per minute on at least 2 of the 13 physical exams. One dog in each of the 1X and 3X groups developed gallop heart sounds after treatment began that were noted in 12 of 13 and 6 of 13 physical exams respectively. Dogs in the PPA-treated group exhibited anxious/ restless behavior more frequently than the control group. One dog each in the 1X and 3X were responsible for the majority of the observations. A decline in mean body weight and body condition was observed in females in all 4 groups, including the control. One female in the 1X group lost 33% body weight. Vomiting and loose stool occurred in a dose-related fashion, and most of the vomiting episodes took place within 1 hour of dosing. Mean platelet counts were higher in at least one of the PPA treated groups, with individual values up to 1.4X the upper limit of normal (ULN) in the 3X and 5X group. The 3X and 5X groups had higher mean serum ALT values compared to the control. Mean ALT was within the normal range for all 4 groups. There were more dogs with ALT levels above the normal range in the 3X PPA treated compared to the control, but increased values were transient and less than 1.8X ULN. All dogs had ALT values in the normal range at the conclusion of the study.
Tolerance Study (PROIN Chewable Tablets, NADA 141-324)
In a separate tolerance study, 6 healthy female Beagle dogs were administered PROIN at 20 mg/kg body weight (10 times the recommended dose) twice daily for 21 consecutive days. Mean systolic blood pressure was increased in the 10X group compared to the control, but mean values were within the normal range for both groups. Mean diastolic pressures were above the normal range on days 7 and 21 for the 10X group, and day 14 for the control. The 10X dogs had hypertensive MAP values on days 7 and 21, whereas the control dog mean MAP values were in the normal range. There was a trend in 10X dogs for lower heart rates following initiation of PPA treatment. Four of 6 dogs in the 10X group had heart rates below the normal range on day 7, whereas none of the control dogs did. The 10X group dogs had increased hematocrit, hemoglobin, RBC counts, urine specific gravity, and water intake consistent with transient, sub-clinical dehydration that occurred shortly after PPA treatment was started. All 6 dogs in the 10X group vomited at least once during the treatment period, whereas only 1 of the control dogs did. Most of the vomiting episodes took place within 1 hour of dosing. Mean platelet counts were also higher in the 10X dogs on all 3 exam days; mean values were above the normal range on day 7, with individual values up to 1.5X ULN. The 10X group had a higher mean serum ALT value on day 7 than the control. Mean ALT values for both groups were in the normal range on all 3 exam days, but 2 dogs in the 10X group had ALT values up to 1.4X ULN on day 7; these elevated values were transient, and all dogs had normal ALT values on days 14 and 21.
For either study, there was no evidence of chronic hypertension-induced target organ damage; there were no clinical findings attributed to PPA on the ophthalmic exams, electrocardiogram evaluation, or gross necropsy and histopathology.
- STORAGE:
- HOW SUPPLIED:
-
REFERENCES:
1 Watson R, et. al. Ephedra alkaloids inhibit platelet aggregation. Blood Coagulation and Fibrinolysis. 2010 21: 266-271
2 Richter K. P., Ling G.V. Clinical response and urethral pressure profile changes after phenylpropanolamine in dogs with primary sphincter incompetence. JAVMA Vol 187 No. 6, September 15, 1985, 605-611.
3 Scott, L., Leddy M., and Bernay F. Evaluation of phenylpropanolamine in the treatment of urethral sphincter mechanism incompetence in the bitch. J. Small Animal Pract. 2002; 43 (11): 493-6.
4 Noel, S., et. al. Combined pharmacokinetic and urodynamic study of the effects of oral administration of phenylpropanolamine in female Beagle dogs. Vet Journal, 2010; 184 (2): 201-207.
- SPL UNCLASSIFIED SECTION
-
Principal Display Panel:
PROIN ERTM
(phenylpropanolamine hydrochloride extended-release tablets)
74 mg / for dogs 41-80 lbs. of body weight
For oral use in dogs only
Caution: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Sympathomimetic amine drug
Approved by FDA under NADA # 141- 517
NDC #: 49427-346-57 or 49427-346-44
PRN Pharmacal
NET CONTENTS 30 or 90 TABLETS

Add image transcription here...

Add image transcription here...
-
INGREDIENTS AND APPEARANCE
PROIN ER
phenylpropanolamine hydrochloride tablet, extended releaseProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:49427-346 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PHENYLPROPANOLAMINE HYDROCHLORIDE (UNII: 8D5I63UE1Q) (PHENYLPROPANOLAMINE - UNII:33RU150WUN) PHENYLPROPANOLAMINE HYDROCHLORIDE 74 mg Product Characteristics Color brown Score no score Shape ROUND Size 12mm Flavor LIVER Imprint Code 74 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49427-346-57 30 in 1 BOTTLE 2 NDC:49427-346-44 90 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141517 10/15/2019 Labeler - Pegasus Laboratories, Inc. (108454760) Registrant - Pegasus Laboratories, Inc. (108454760) Establishment Name Address ID/FEI Business Operations Pegasus Laboratories, Inc. 108454760 manufacture, analysis, label


