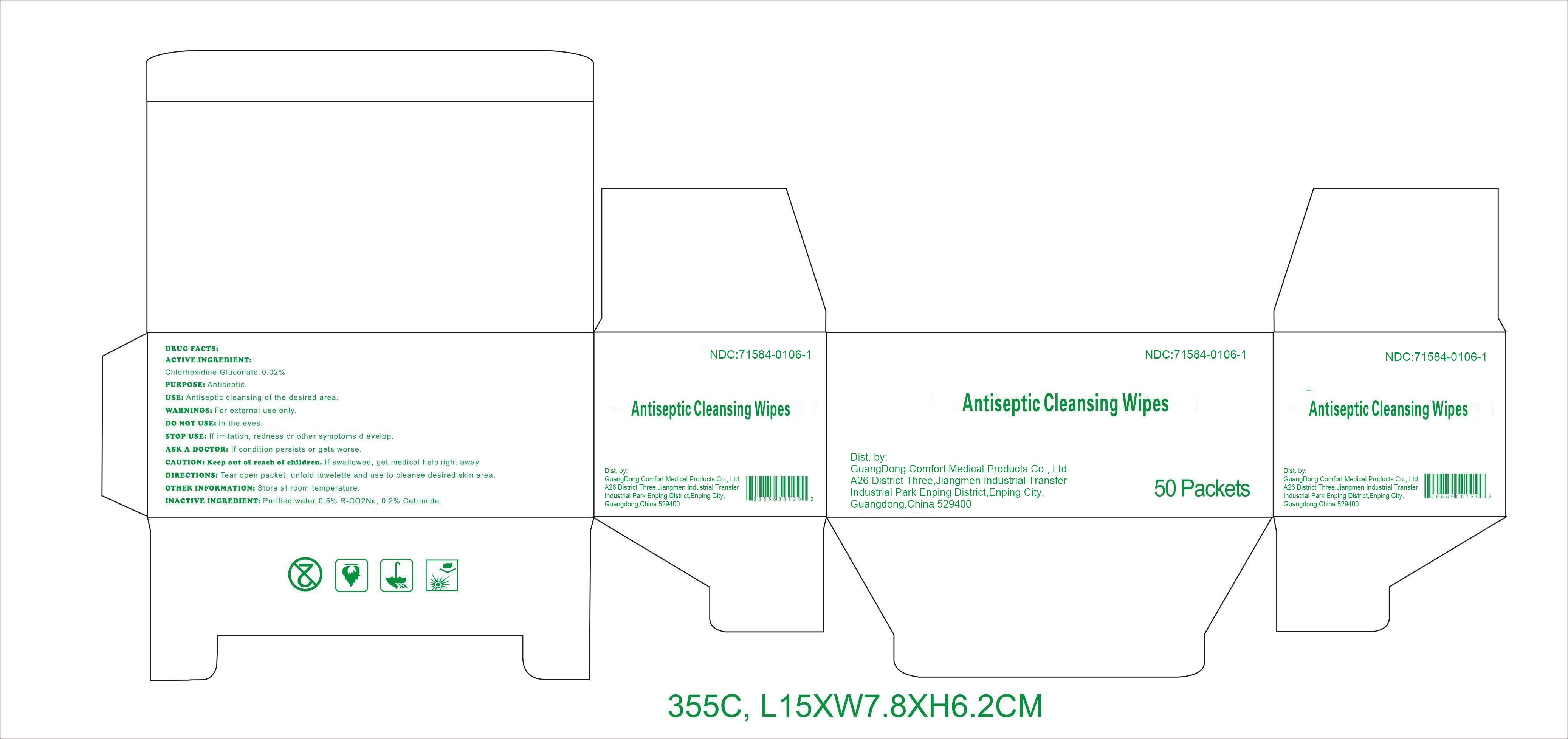
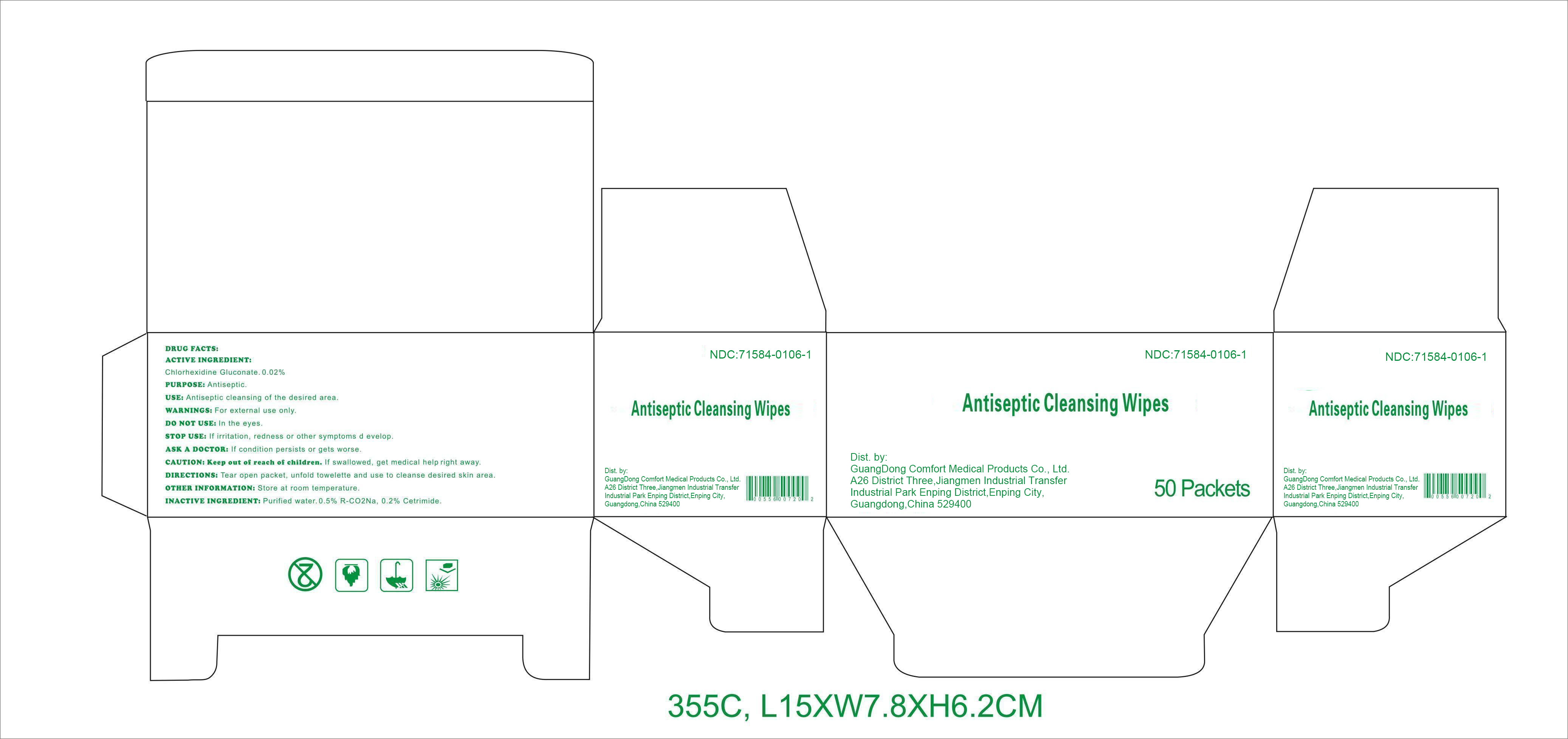
Label: ANTISEPTIC CLEANSING WIPES- chlorhexidine gluconate swab
-
Contains inactivated NDC Code(s)
NDC Code(s): 71584-0106-1 - Packager: Guangdong Comfort Medical Products Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated October 18, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- CAUTION: Keep out of reach of children
- Use
- Warnings
- Directions
- Inactive ingredients
-
DRUG FACTS
Active ingredient
Chlorhexidine Gluconate 0.02%
Purpose
Antiseptic
Use
Antiseptic cleansing of the desired area.
Warnings
For external use only.
Do notuse
﹒in the eyes.
Stop use
If irritation, redness or other symptoms develop.
Ask a doctor
If condition persists or gets worse.
CAUTION: Keep out of reach of children.
If swallowed, get medical help right away.
Directions
Tear open packet, unfold towelette, and use to cleanse desired skin area.
Other information: Store at room temperature.
Inactive ingredients
Purified water, 0.5% R-CO 2Na, 0.2% Cetrimide

-
INGREDIENTS AND APPEARANCE
ANTISEPTIC CLEANSING WIPES
chlorhexidine gluconate swabProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71584-0106 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORHEXIDINE GLUCONATE (UNII: MOR84MUD8E) (CHLORHEXIDINE - UNII:R4KO0DY52L) CHLORHEXIDINE GLUCONATE 0.0002 mg in 1 mg Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CETRIMIDE (UNII: 24QSH2NL8N) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71584-0106-1 1.2 mg in 1 PACKET; Type 0: Not a Combination Product 07/17/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 07/17/2017 Labeler - Guangdong Comfort Medical Products Co., Ltd. (544507534) Registrant - Guangdong Comfort Medical Products Co., Ltd. (544507534)