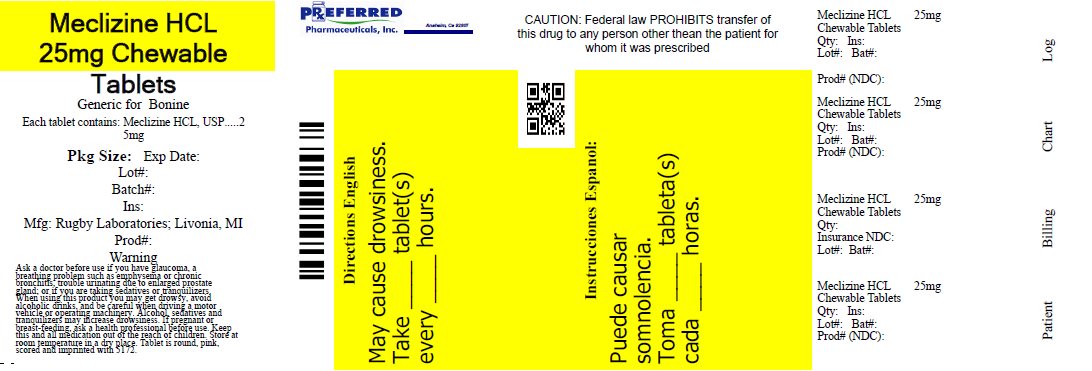
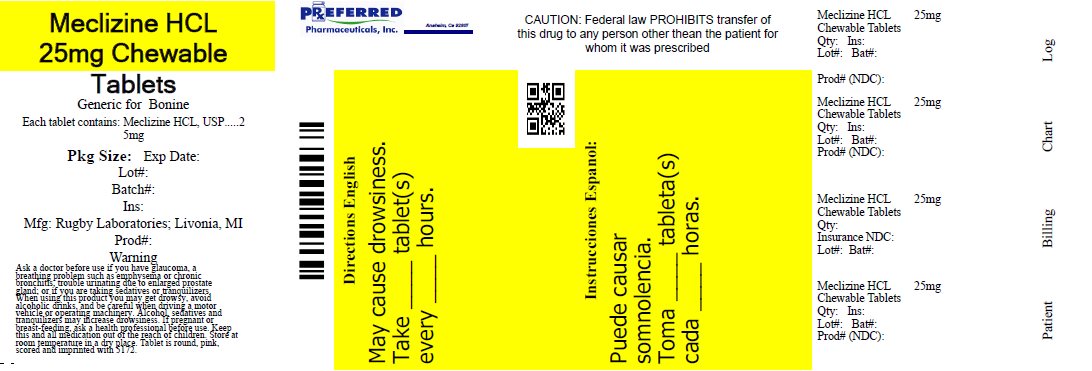
Label: MECLIZINE HYDROCHLORIDE tablet, chewable
- NDC Code(s): 68788-7873-1, 68788-7873-2, 68788-7873-3
- Packager: Preferred Pharmaceuticals Inc.
- This is a repackaged label.
- Source NDC Code(s): 0536-1299
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated July 2, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- PURPOSE
- Uses
-
WARNINGS
Ask a doctor before use if you have
glaucoma
a breathing problem such as emphysema or chronic bronchitis
trouble urinating due to an enlarged prostate gland - Directions
- Other information
- INACTIVE INGREDIENT
- Questions or comments?
-
SPL UNCLASSIFIED SECTION
THIS PACKAGE FOR HOUSEHOLDS WITHOUT YOUNG CHILDREN
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
*This product is not manufactured or distributed by Prestige Brands, Inc owner of the registered trademark Bonine®.
Distributed by:
RUGBY® LABORATORIES
17177 N Laurel Park Dr., Suite 233
Livonia, MI 48152
www.rugbylaboratories.comRepackaged By: Preferred Pharmaceuticals Inc.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MECLIZINE HYDROCHLORIDE
meclizine hydrochloride tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68788-7873(NDC:0536-1299) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MECLIZINE HYDROCHLORIDE (UNII: HDP7W44CIO) (MECLIZINE - UNII:3L5TQ84570) MECLIZINE HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength CROSPOVIDONE, UNSPECIFIED (UNII: 2S7830E561) VANILLA BEAN (UNII: Q74T35078H) MAGNESIUM STEARATE (UNII: 70097M6I30) RASPBERRY (UNII: 4N14V5R27W) STEARIC ACID (UNII: 4ELV7Z65AP) FD&C RED NO. 40 (UNII: WZB9127XOA) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) SACCHARIN SODIUM (UNII: SB8ZUX40TY) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Product Characteristics Color pink (Rosy) Score 2 pieces Shape ROUND Size 9mm Flavor VANILLA, RASPBERRY Imprint Code 5172 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68788-7873-1 100 in 1 BOTTLE; Type 0: Not a Combination Product 02/19/2021 2 NDC:68788-7873-3 1000 in 1 BOTTLE; Type 0: Not a Combination Product 02/19/2021 08/26/2022 3 NDC:68788-7873-2 30 in 1 BOTTLE; Type 0: Not a Combination Product 08/26/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M009 02/19/2021 Labeler - Preferred Pharmaceuticals Inc. (791119022) Registrant - Preferred Pharmaceuticals Inc. (791119022) Establishment Name Address ID/FEI Business Operations Preferred Pharmaceuticals Inc. 791119022 REPACK(68788-7873)