Label: CHLORPHENIRAMINE MALEATE 4 MG- chlorpheniramine maleate tablet
-
NDC Code(s):
71335-0963-1,
71335-0963-2,
71335-0963-3,
71335-0963-4, view more71335-0963-5, 71335-0963-6
- Packager: Bryant Ranch Prepack
- This is a repackaged label.
- Source NDC Code(s): 69618-022
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated May 17, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient (in each tablet)
- Purpose
- Warnings
- ASK DOCTOR/PHARMACIST
- GENERAL PRECAUTIONS
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
- Uses
-
HOW SUPPLIED
NDC: 71335-0963-1: 30 Tablets in a BOTTLE
NDC: 71335-0963-2: 60 Tablets in a BOTTLE
NDC: 71335-0963-3: 100 Tablets in a BOTTLE
NDC: 71335-0963-4: 120 Tablets in a BOTTLE
NDC: 71335-0963-5: 40 Tablets in a BOTTLE
NDC: 71335-0963-6: 24 Tablets in a BOTTLE
Repackaged/Relabeled by:
Bryant Ranch Prepack, Inc.
Burbank, CA 91504 - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CHLORPHENIRAMINE MALEATE 4 MG
chlorpheniramine maleate tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71335-0963(NDC:69618-022) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORPHENIRAMINE MALEATE (UNII: V1Q0O9OJ9Z) (CHLORPHENIRAMINE - UNII:3U6IO1965U) CHLORPHENIRAMINE MALEATE 4 mg Inactive Ingredients Ingredient Name Strength D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) STARCH, CORN (UNII: O8232NY3SJ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Product Characteristics Color yellow Score no score Shape ROUND Size 8mm Flavor Imprint Code AP;016 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71335-0963-1 30 in 1 BOTTLE; Type 0: Not a Combination Product 10/05/2018 2 NDC:71335-0963-2 60 in 1 BOTTLE; Type 0: Not a Combination Product 05/17/2024 3 NDC:71335-0963-3 100 in 1 BOTTLE; Type 0: Not a Combination Product 05/17/2024 4 NDC:71335-0963-4 120 in 1 BOTTLE; Type 0: Not a Combination Product 05/17/2024 5 NDC:71335-0963-5 40 in 1 BOTTLE; Type 0: Not a Combination Product 05/17/2024 6 NDC:71335-0963-6 24 in 1 BOTTLE; Type 0: Not a Combination Product 05/17/2024 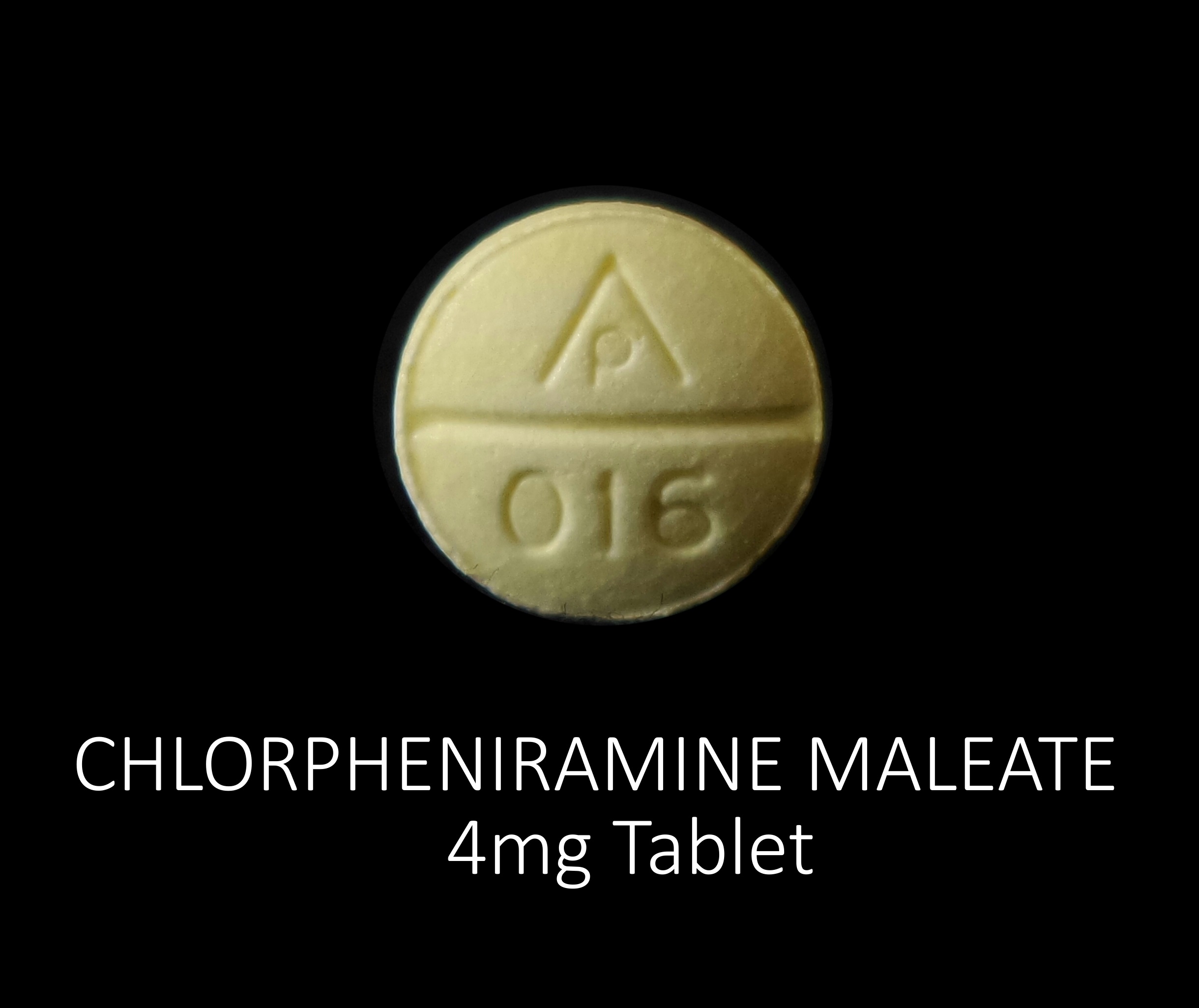
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 11/01/2015 Labeler - Bryant Ranch Prepack (171714327) Registrant - Bryant Ranch Prepack (171714327) Establishment Name Address ID/FEI Business Operations Bryant Ranch Prepack 171714327 REPACK(71335-0963) , RELABEL(71335-0963)


