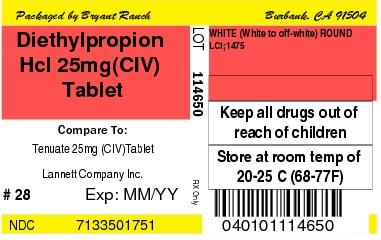
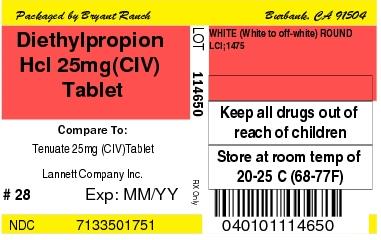
Label: DIETHYLPROPION HYDROCHLORIDE tablet
-
NDC Code(s):
71335-0175-0,
71335-0175-1,
71335-0175-2,
71335-0175-3, view more71335-0175-4, 71335-0175-5, 71335-0175-6, 71335-0175-7, 71335-0175-8, 71335-0175-9
- Packager: Bryant Ranch Prepack
- This is a repackaged label.
- Source NDC Code(s): 0527-1475
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CIV
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 19, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Diethylpropion Hydrochloride Tablets USP, 25 mg are available for oral administration as tablets containing 25 mg diethylpropion hydrochloride, USP. The inactive ingredients in each tablet are: microcrystalline cellulose, lactose monohydrate, mannitol, tartaric acid, povidone, talc, and zinc stearate.
Diethylpropion hydrochloride is a sympathomimetic agent. The chemical name for diethylpropion hydrochloride is 1-phenyl-2-diethyl-amino-1-propanone hydrochloride.
Its chemical structure is:

-
CLINICAL PHARMACOLOGY
Diethylpropion hydrochloride is a sympathomimetic amine with some pharmacologic activity similar to that of the prototype drugs of this class used in obesity, the amphetamines. Actions include some central nervous system stimulation and elevation of blood pressure. Tolerance has been demonstrated with all drugs of this class in which these phenomena have been looked for.
Drugs of this class used in obesity are commonly known as "anorectics" or "anorexigenics". It has not been established, however, that the action of such drugs in treating obesity is primarily one of appetite suppression. For example, other central nervous system actions or metabolic effects may be involved.
Adult obese subjects instructed in dietary management and treated with "anorectic" drugs lose more weight on the average than those treated with placebo and diet, as determined in relatively short-term clinical trials.
The magnitude of increased weight loss of drug-treated patients over placebo-treated patients averages some fraction of a pound a week. However, individual weight loss may vary substantially from patient to patient. The rate of weight loss is greatest in the first weeks of therapy for both drug and placebo subjects and tends to decrease in succeeding weeks. The possible origins of the increased weight loss due to the various drug effects are not established. The amount of weight loss associated with the use of an "anorectic" drug varies from trial to trial, and the increased weight loss appears to be related in part to variables other than the drug prescribed, such as the physician/investigator relationship, the population treated, and the diet prescribed. Studies do not permit conclusions as to the relative importance of the drug and non-drug factors on weight loss.
The natural history of obesity is measured in years, whereas most studies cited are restricted to a few weeks duration; thus, the total impact of drug-induced weight loss over that of diet alone is unknown.
Diethylpropion is rapidly absorbed from the GI tract after oral administration and is extensively metabolized through a complex pathway of biotransformation involving N-dealkylation and reduction. Many of these metabolites are biologically active and may participate in the therapeutic action of diethylpropion hydrochloride tablets USP, 25 mg. Due to the varying lipid solubilities of these metabolites, their circulating levels are affected by urinary pH. Diethylpropion and/or its active metabolites are believed to cross the blood-brain barrier and the placenta.
Diethylpropion and its metabolites are excreted mainly by the kidney. It has been reported that between 75-106% of the dose is recovered in the urine within 48 hours after dosing. Using a phosphorescence assay that is specific for basic compounds containing benzoyl group, the plasma half-life of the aminoketone metabolites is estimated to be between 4 to 6 hours.
-
INDICATIONS AND USAGE
Diethylpropion hydrochloride tablets USP, 25 mg are indicated in the management of exogenous obesity as a short-term adjunct (a few weeks) in a regimen of weight reduction based on caloric restriction in patients with an initial body mass index (BMI) of 30 kg/m2 or higher and who have not responded to appropriate weight reducing regimen (diet and/or exercise) alone. Below is a chart of BMI based on various heights and weights. BMI is calculated by taking the patient's weight, in kilograms (kg), divided by the patient's height, in meters (m), squared. Metric conversions are as follows: pounds divided by 2.2 = kg; inches × 0.0254 = meters.
Body Mass Index (BMI), kg/m2 Weight (pounds) Height (feet, inches) 5'0" 5'3" 5'6" 5'9" 6'0" 6'3" 140 27 25 23 21 19 18 150 29 27 24 22 20 19 160 31 28 26 24 22 20 170 33 30 28 25 23 21 180 35 32 29 27 25 23 190 37 34 31 28 26 24 200 39 36 32 30 27 25 210 41 37 34 31 29 26 220 43 39 36 33 30 28 230 45 41 37 34 31 29 240 47 43 39 36 33 30 250 49 44 40 37 34 31 The usefulness of agents of this class (See CLINICAL PHARMACOLOGY) should be measured against possible risk factors inherent in their use such as those described below. Diethylpropion hydrochloride tablets USP, 25 mg are indicated for use as monotherapy only.
-
CONTRAINDICATIONS
Pulmonary hypertension, advanced arteriosclerosis, hyperthyroidism, known hypersensitivity or idiosyncrasy to the sympathomimetic amines, glaucoma, severe hypertension (See PRECAUTIONS).
Agitated states.
Patients with a history of drug abuse.
Use in combination with other anorectic agents is contraindicated.
During or within 14 days following the administration of monoamine oxidase inhibitors, hypertensive crises may result.
-
WARNINGS
Diethylpropion hydrochloride tablets USP, 25 mg should not be used in combination with other anorectic agents, including prescribed drugs, over-the-counter preparations, and herbal products.
In a case-control epidemiological study, the use of anorectic agents, including diethylpropion, was associated with an increased risk of developing pulmonary hypertension, a rare, but often fatal disorder. The use of anorectic agents for longer than 3 months was associated with a 23-fold increase in the risk of developing pulmonary hypertension. Increased risk of pulmonary hypertension with repeated courses of therapy cannot be excluded.
The onset or aggravation of exertional dyspnea, or unexplained symptoms of angina pectoris, syncope, or lower extremity edema suggest the possibility of occurrence of pulmonary hypertension. Under these circumstances, diethylpropion hydrochloride tablets USP, 25 mg should be immediately discontinued, and the patient should be evaluated for the possible presence of pulmonary hypertension.
Valvular heart disease associated with the use of some anorectic agents such as fenfluramine and dexfenfluramine has been reported. Possible contributing factors include use for extended periods of time, higher than recommended dose, and/or use in combination with other anorectic drugs. Valvulopathy has been very rarely reported with diethylpropion hydrochloride tablets USP, 25 mg monotherapy, but the causal relationship remains uncertain. The potential risk of possible serious adverse effects such as valvular heart disease and pulmonary hypertension should be assessed carefully against the potential benefit of weight loss. Baseline cardiac evaluation should be considered to detect preexisting valvular heart diseases or pulmonary hypertension prior to initiation of diethylpropion hydrochloride tablets USP, 25 mg treatment. Diethylpropion hydrochloride tablets USP, 25 mg are not recommended in patients with known heart murmur or valvular heart disease. Echocardiogram during and after treatment could be useful for detecting any valvular disorders which may occur.
To limit unwarranted exposure and risks, treatment with diethylpropion hydrochloride tablets USP, 25 mg should be continued only if the patient has satisfactory weight loss within the first 4 weeks of treatment (e.g., weight loss of at least 4 pounds, or as determined by the physician and patient).
Diethylpropion hydrochloride tablets USP, 25 mg are not recommended for patients who used any anorectic agents within the prior year.
If tolerance develops, the recommended dose should not be exceeded in an attempt to increase the effect; rather, the drug should be discontinued. Diethylpropion hydrochloride tablets USP, 25 mg may impair the ability of the patient to engage in potentially hazardous activities such as operating machinery or driving a motor vehicle; the patient should therefore be cautioned accordingly.
Prolonged use of diethylpropion hydrochloride may induce dependence with withdrawal syndrome on cessation of therapy. Hallucinations have occurred rarely following high doses of the drug. Several cases of toxic psychosis have been reported following the excessive use of the drug and some have been reported in which the recommended dose appears not to have been exceeded. Psychosis abated after the drug was discontinued.
When central nervous system active agents are used, consideration must always be given to the possibility of adverse interactions with alcohol.
-
PRECAUTIONS
General
Caution is to be exercised in prescribing diethylpropion hydrochloride tablets USP, 25 mg for patients with hypertension or with symptomatic cardiovascular disease, including arrhythmias. Diethylpropion hydrochloride tablets USP, 25 mg should not be administered to patients with severe hypertension.
Reports suggest that diethylpropion hydrochloride may increase convulsions in some epileptics. Therefore, epileptics receiving diethylpropion hydrochloride tablets USP, 25 mg should be carefully monitored. Titration of dose or discontinuance of diethylpropion hydrochloride tablets USP, 25 mg may be necessary.
The least amount feasible should be prescribed or dispensed at one time in order to minimize the possibility of overdosage.
Information for Patient
The patient should be cautioned about concomitant use of alcohol or other CNS-active drugs and diethylpropion hydrochloride tablets USP, 25 mg (See WARNINGS). The patient should be advised to observe caution when driving or engaging in any potentially hazardous activity.
Drug Interactions
Because diethylpropion hydrochloride tablets USP, 25 mg are monoamines, hypertension may result when either agent is used with monoamine oxidase (MAO) inhibitors (See CONTRAINDICATIONS).
Efficacy of diethylpropion with other anorectic agents has not been studied and the combined use may have the potential for serious cardiac problems; therefore, the concomitant use with other anorectic agents is contraindicated.
Antidiabetic drug requirements (i.e., insulin) may be altered. Concurrent use with general anesthetics may result in arrhythmias. The pressor effects of diethylpropion and those of other drugs may be additive when the drugs are used concomitantly; conversely, diethylpropion may interfere with antihypertensive drugs (i.e., guanethidine, a-methyldopa). Concurrent use of phenothiazines may antagonize the anorectic effect of diethylpropion.
Carcinogenesis, Mutagenesis, and Impairment of Fertility
No long-term animal studies have been done to evaluate diethylpropion hydrochloride for carcinogenicity. Mutagenicity studies have not been conducted. Animal reproduction studies have revealed no evidence of impairment of fertility (See Pregnancy).
Pregnancy
Teratogenic Effects: Pregnancy Category B. Reproduction studies have been performed in rats at doses up to 1.6 times the human dose (based on mg/m2) and have revealed no evidence of impaired fertility or harm to the fetus due to diethylpropion hydrochloride. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Spontaneous reports of congenital malformations have been recorded in humans, but no causal relationship to diethylpropion has been established.
Non-Teratogenic Effects. Abuse with diethylpropion hydrochloride during pregnancy may result in withdrawal symptoms in the human neonate.
Nursing Mothers
Since diethylpropion hydrochloride and/or its metabolites have been shown to be excreted in human milk, caution should be exercised when diethylpropion hydrochloride tablets USP, 25 mg are administered to a nursing woman.
Geriatric Use
Clinical studies of diethylpropion hydrochloride tablets USP, 25 mg did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects.
Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
-
ADVERSE REACTIONS
Cardiovascular: Precordial pain, arrhythmia (including ventricular), ECG changes, tachycardia, elevation of blood pressure, palpitation and rare reports of pulmonary hypertension. Valvular heart disease associated with the use of some anorectic agents such as fenfluramine and dexfenfluramine, both independently and especially when used in combination, have been reported. Valvulopathy has been very rarely reported with diethylpropion hydrochloride tablets USP, 25 mg monotherapy, but the causal relationship remains uncertain.
Central Nervous System: In a few epileptics an increase in convulsive episodes has been reported; rarely psychotic episodes at recommended doses; dyskinesia, blurred vision, overstimulation, nervousness, restlessness, dizziness, jitteriness, insomnia, anxiety, euphoria, depression, dysphoria, tremor, mydriasis, drowsiness, malaise, headache, and cerebrovascular accident
Gastrointestinal: Vomiting, diarrhea, abdominal discomfort, dryness of the mouth, unpleasant taste, nausea, constipation, other gastrointestinal disturbances
Allergic: Urticaria, rash, ecchymosis, erythema
Endocrine: Impotence, changes in libido, gynecomastia, menstrual upset
Hematopoietic System: Bone marrow depression, agranulocytosis, leukopenia
Miscellaneous: A variety of miscellaneous adverse reactions has been reported by physicians. These include complaints such as dysuria, dyspnea, hair loss, muscle pain, increased sweating, and polyuria.
-
DRUG ABUSE AND DEPENDENCE
Diethylpropion hydrochloride tablets USP, 25 mg are schedule IV controlled substances. Diethylpropion hydrochloride has some chemical and pharmacologic similarities to the amphetamines and other related stimulant drugs that have been extensively abused. There have been reports of subjects becoming psychologically dependent on diethylpropion. The possibility of abuse should be kept in mind when evaluating the desirability of including a drug as part of a weight reduction program. Abuse of amphetamines and related drugs may be associated with varying degrees of psychologic dependence and social dysfunction which, in the case of certain drugs, may be severe. There are reports of patients who have increased the dosage to many times that recommended. Abrupt cessation following prolonged high dosage administration results in extreme fatigue and mental depression; changes are also noted on the sleep EEG. Manifestations of chronic intoxication with anorectic drugs include severe dermatoses, marked insomnia, irritability, hyperactivity, and personality changes. The most severe manifestation of chronic intoxication is psychosis, often clinically indistinguishable from schizophrenia.
-
OVERDOSAGE
Manifestations of acute overdosage include restlessness, tremor, hyperreflexia, rapid respiration, confusion, assaultiveness, hallucinations, panic states, and mydriasis. Fatigue and depression usually follow the central stimulation.
Cardiovascular effects include tachycardia, arrhythmias, hypertension or hypotension and circulatory collapse. Gastrointestinal symptoms include nausea, vomiting, diarrhea, and abdominal cramps. Overdose of pharmacologically similar compounds has resulted in convulsions, coma and death.
The reported oral LD50 for mice is 600 mg/kg, for rats is 250 mg/kg and for dogs is 225 mg/kg.
Management of acute diethylpropion hydrochloride intoxication is largely symptomatic and includes lavage and sedation with a barbiturate. Experience with hemodialysis or peritoneal dialysis is inadequate to permit recommendation in this regard. Intravenous phentolamine has been suggested on pharmacologic grounds for possible acute, severe hypertension, if this complicates diethylpropion hydrochloride tablets USP, 25 mg overdosage.
-
DOSAGE AND ADMINISTRATION
Diethylpropion Hydrochloride Tablets USP, 25 mg:
One 25 mg tablet three times daily, one hour before meals, and in midevening if desired to overcome night hunger.Geriatric use:
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function (See PRECAUTIONS, Geriatric Use).
-
HOW SUPPLIED
Product: 71335-0175
NDC: 71335-0175-0 42 TABLET in a BOTTLE
NDC: 71335-0175-1 28 TABLET in a BOTTLE
NDC: 71335-0175-2 90 TABLET in a BOTTLE
NDC: 71335-0175-3 30 TABLET in a BOTTLE
NDC: 71335-0175-4 14 TABLET in a BOTTLE
NDC: 71335-0175-5 21 TABLET in a BOTTLE
NDC: 71335-0175-6 7 TABLET in a BOTTLE
NDC: 71335-0175-7 60 TABLET in a BOTTLE
NDC: 71335-0175-8 84 TABLET in a BOTTLE
NDC: 71335-0175-9 56 TABLET in a BOTTLE
- Diethylpropion Hcl 25mg(CIV) Tablet
-
INGREDIENTS AND APPEARANCE
DIETHYLPROPION HYDROCHLORIDE
diethylpropion hydrochloride tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:71335-0175(NDC:0527-1475) Route of Administration ORAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIETHYLPROPION HYDROCHLORIDE (UNII: 19V2PL39NG) (DIETHYLPROPION - UNII:Q94YYU22B8) DIETHYLPROPION HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength TARTARIC ACID (UNII: W4888I119H) MANNITOL (UNII: 3OWL53L36A) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) TALC (UNII: 7SEV7J4R1U) ZINC STEARATE (UNII: H92E6QA4FV) Product Characteristics Color WHITE (White to off-white) Score no score Shape ROUND (flat-faced beveled edge) Size 10mm Flavor Imprint Code LCI;1475 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71335-0175-2 90 in 1 BOTTLE; Type 0: Not a Combination Product 10/21/2011 2 NDC:71335-0175-5 21 in 1 BOTTLE; Type 0: Not a Combination Product 10/21/2011 3 NDC:71335-0175-8 84 in 1 BOTTLE; Type 0: Not a Combination Product 10/21/2011 4 NDC:71335-0175-0 42 in 1 BOTTLE; Type 0: Not a Combination Product 10/21/2011 5 NDC:71335-0175-3 30 in 1 BOTTLE; Type 0: Not a Combination Product 10/21/2011 6 NDC:71335-0175-9 56 in 1 BOTTLE; Type 0: Not a Combination Product 10/21/2011 7 NDC:71335-0175-6 7 in 1 BOTTLE; Type 0: Not a Combination Product 10/21/2011 8 NDC:71335-0175-7 60 in 1 BOTTLE; Type 0: Not a Combination Product 10/21/2011 9 NDC:71335-0175-1 28 in 1 BOTTLE; Type 0: Not a Combination Product 10/21/2011 10 NDC:71335-0175-4 14 in 1 BOTTLE; Type 0: Not a Combination Product 10/21/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA200177 07/18/2011 Labeler - Bryant Ranch Prepack (171714327) Establishment Name Address ID/FEI Business Operations Bryant Ranch Prepack 171714327 REPACK(71335-0175) , RELABEL(71335-0175)