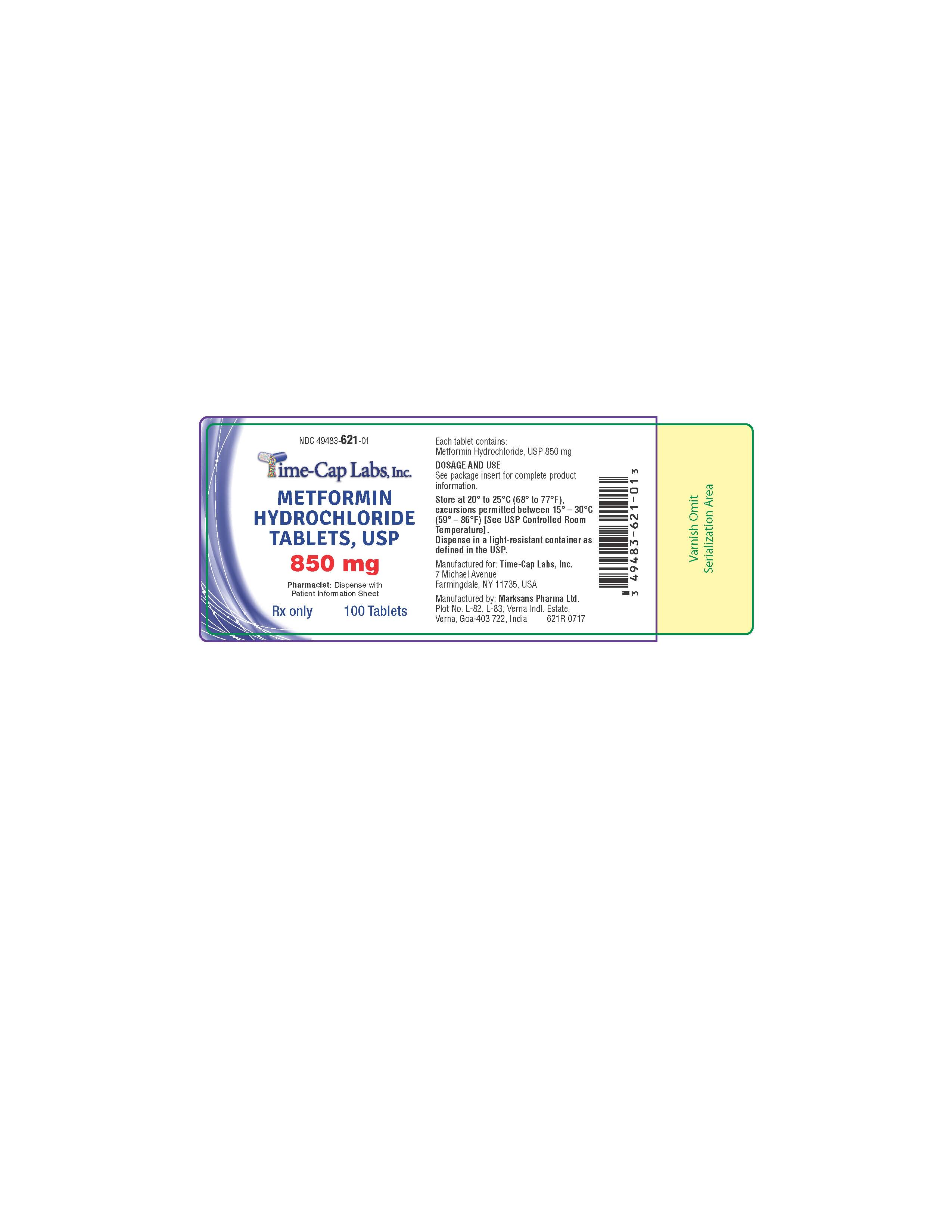
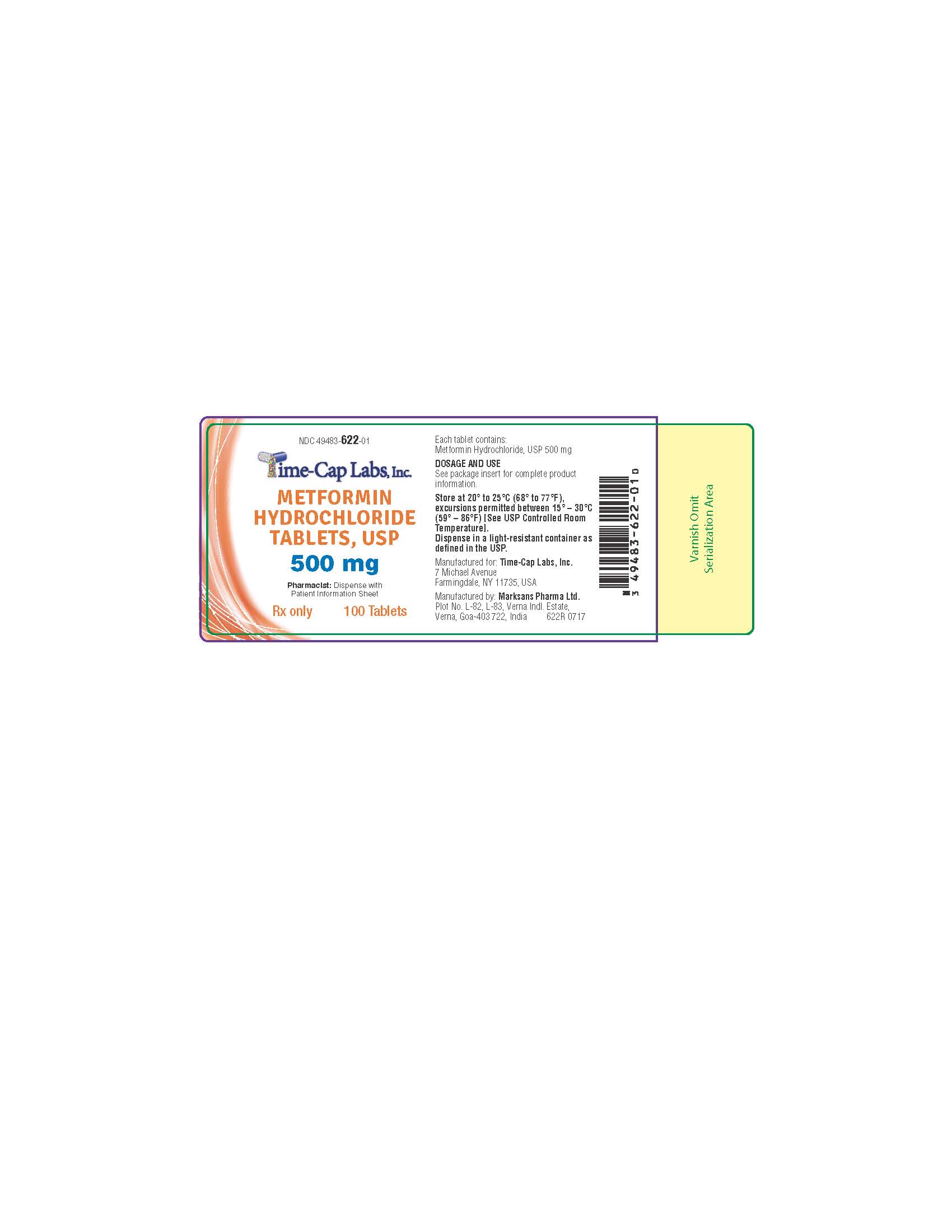
Label: METFORMIN HCL tablet, film coated
-
NDC Code(s):
49483-620-01,
49483-620-10,
49483-620-50,
49483-620-81, view more49483-621-01, 49483-621-10, 49483-621-50, 49483-621-81, 49483-622-01, 49483-622-10, 49483-622-50, 49483-622-81
- Packager: TIME CAP LABORATORIES, INC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 30, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
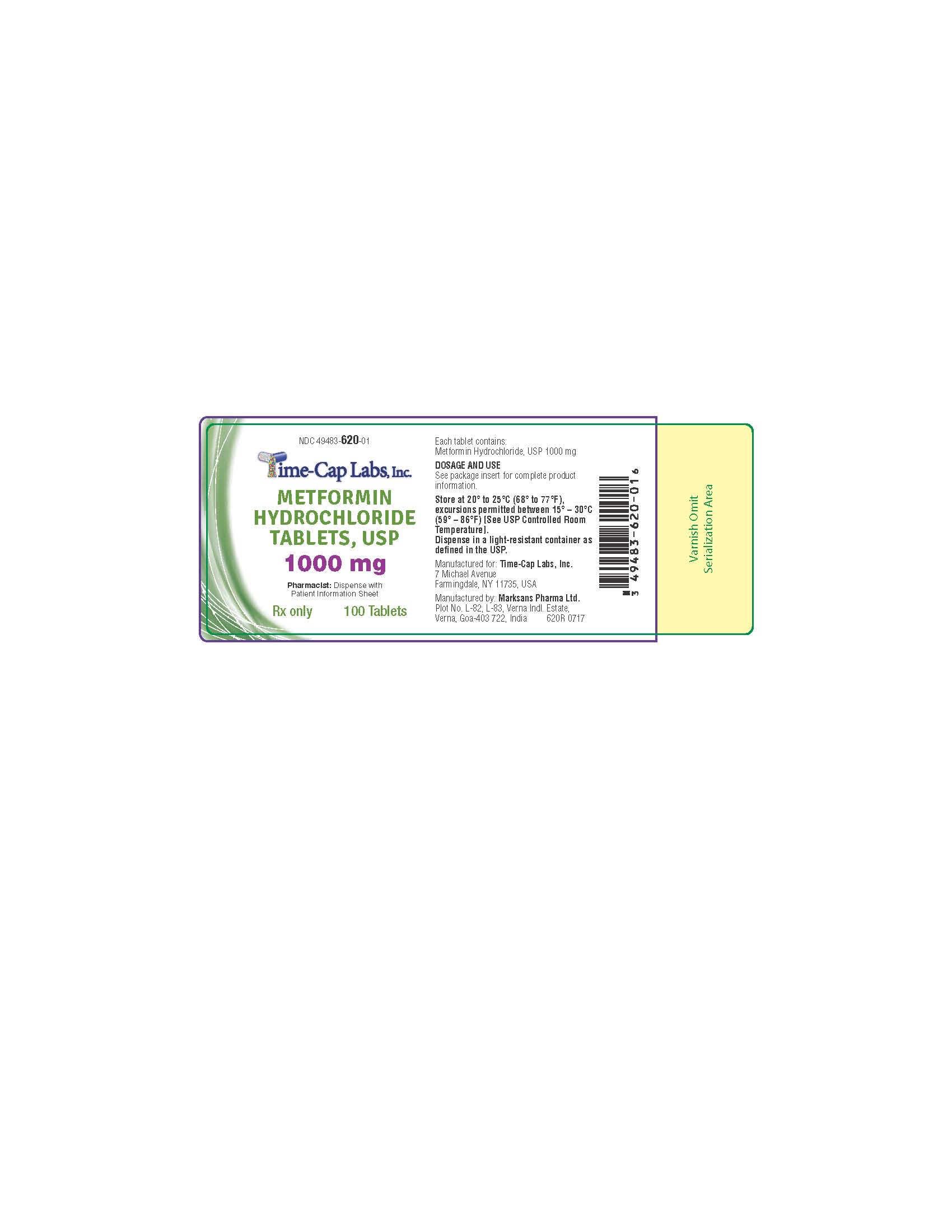
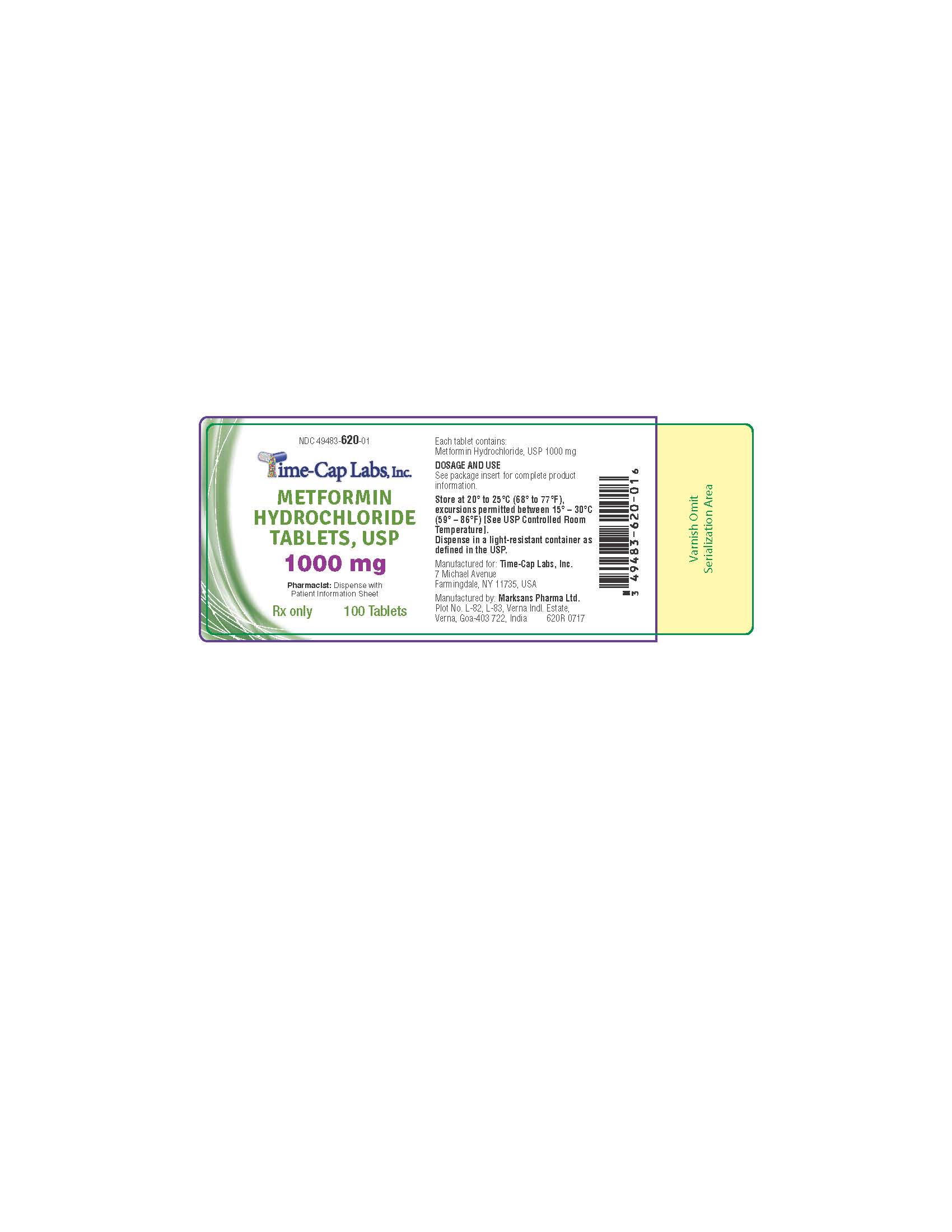
- HOW SUPPLIED
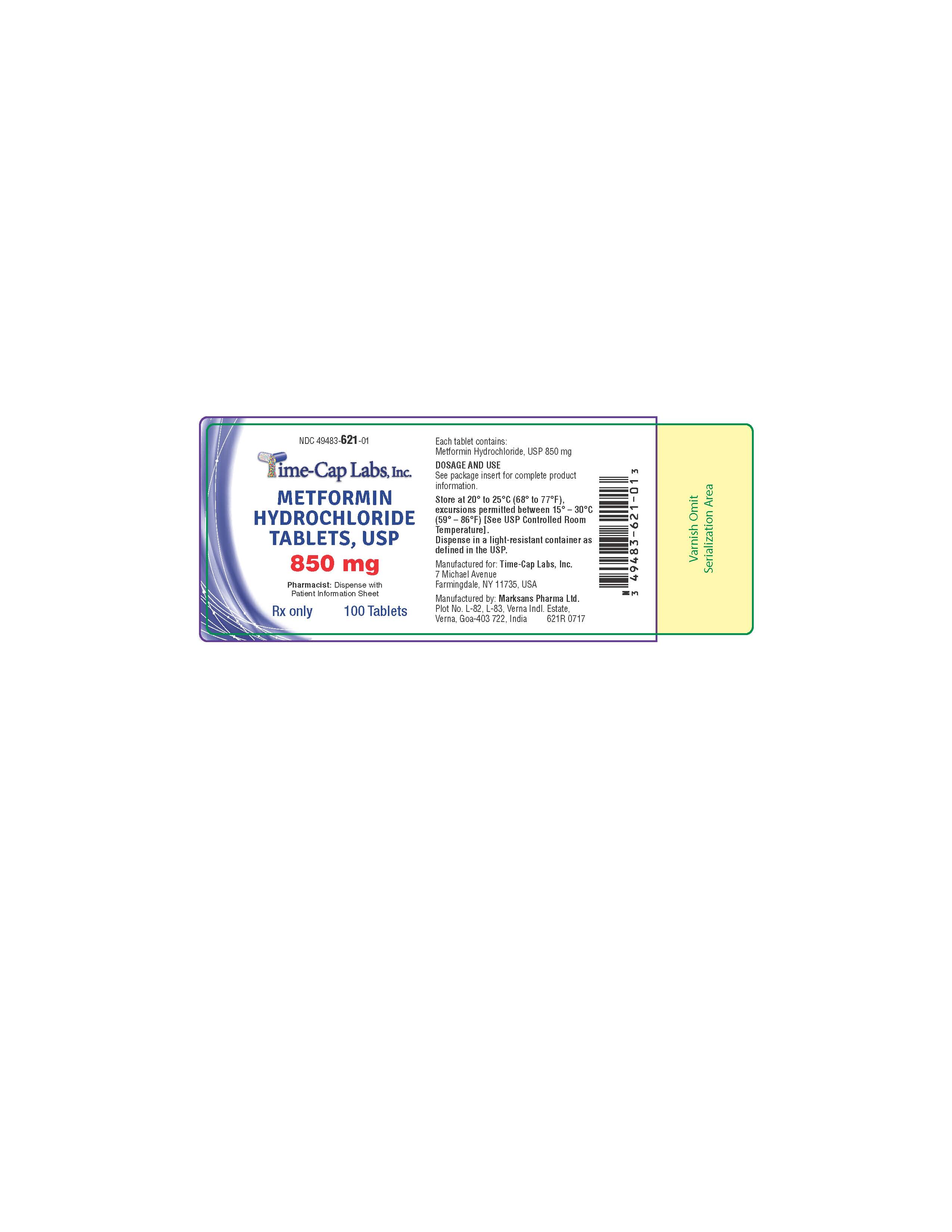
- HOW SUPPLIED
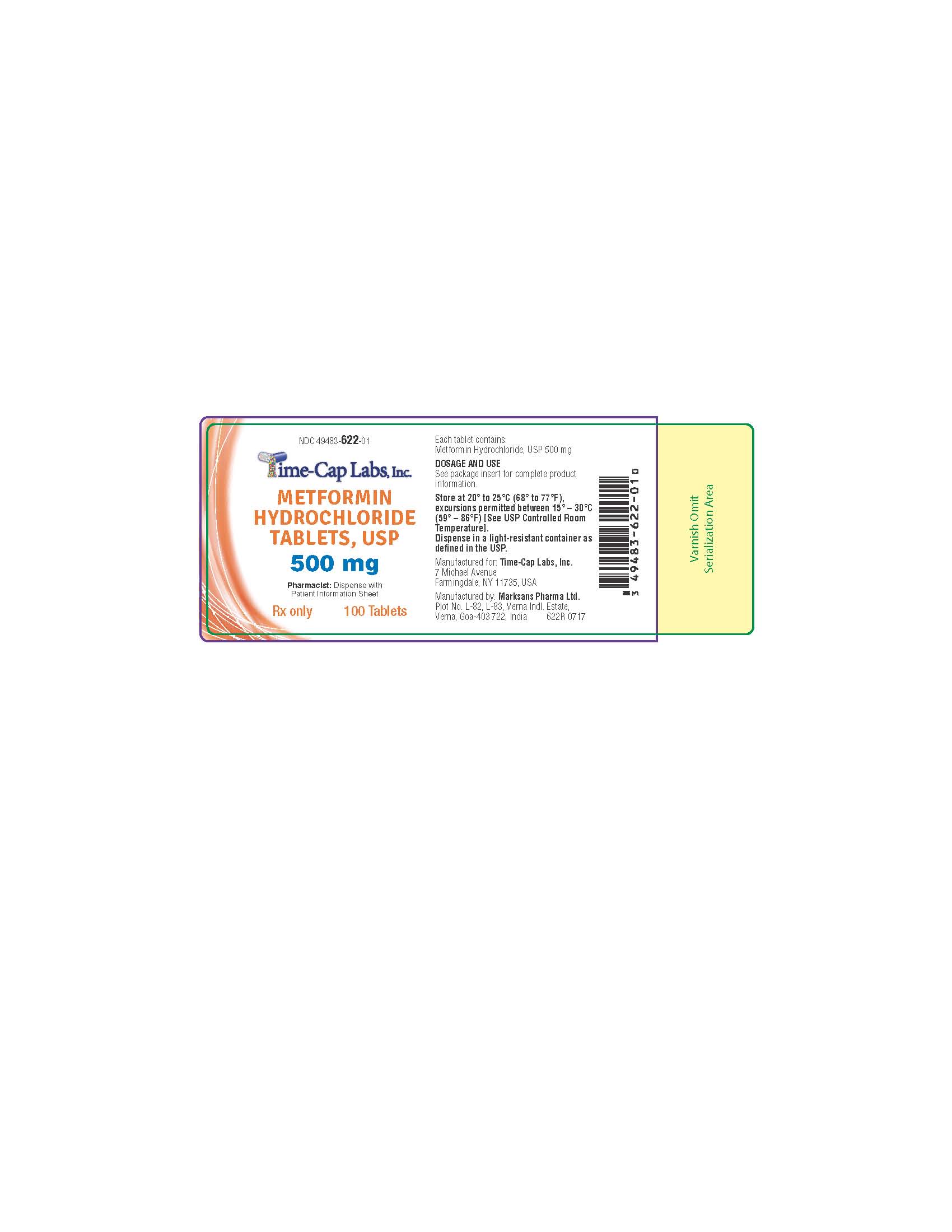
- HOW SUPPLIED
- 100 ct 620R 1000 MG
- 100 CT 621R
- 100 CT 622R
-
INGREDIENTS AND APPEARANCE
METFORMIN HCL
metformin hcl tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:49483-620 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METFORMIN HYDROCHLORIDE (UNII: 786Z46389E) (METFORMIN - UNII:9100L32L2N) METFORMIN HYDROCHLORIDE 1000 mg Inactive Ingredients Ingredient Name Strength POVIDONE (UNII: FZ989GH94E) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color white (WHITE TO OFF-WHITE) Score 2 pieces Shape OVAL Size 19mm Flavor Imprint Code 132 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49483-620-01 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 11/16/2016 2 NDC:49483-620-81 180 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 06/05/2017 3 NDC:49483-620-50 500 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 11/16/2016 4 NDC:49483-620-10 1000 in 1 PACKAGE; Type 0: Not a Combination Product 06/05/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090888 11/14/2016 METFORMIN HCL
metformin hcl tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:49483-621 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METFORMIN HYDROCHLORIDE (UNII: 786Z46389E) (METFORMIN - UNII:9100L32L2N) METFORMIN HYDROCHLORIDE 850 mg Inactive Ingredients Ingredient Name Strength POVIDONE (UNII: FZ989GH94E) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color white (WHITE TO OFF-WHITE) Score no score Shape ROUND Size 13mm Flavor Imprint Code 131 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49483-621-01 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 11/16/2016 2 NDC:49483-621-81 180 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 06/05/2017 3 NDC:49483-621-50 500 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 11/16/2016 4 NDC:49483-621-10 1000 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 06/05/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090888 11/14/2016 METFORMIN HCL
metformin hcl tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:49483-622 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METFORMIN HYDROCHLORIDE (UNII: 786Z46389E) (METFORMIN - UNII:9100L32L2N) METFORMIN HYDROCHLORIDE 500 mg Inactive Ingredients Ingredient Name Strength POVIDONE (UNII: FZ989GH94E) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color white (WHITE TO OFF-WHITE) Score no score Shape ROUND Size 12mm Flavor Imprint Code 134 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49483-622-01 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 11/16/2016 2 NDC:49483-622-81 180 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 06/05/2017 3 NDC:49483-622-50 500 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 11/16/2016 4 NDC:49483-622-10 1000 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 06/05/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090888 11/14/2016 Labeler - TIME CAP LABORATORIES, INC (037052099) Registrant - TIME CAP LABORATORIES, INC (037052099) Establishment Name Address ID/FEI Business Operations MARKSANS PHARMA LIMITED 925822975 manufacture(49483-620, 49483-621, 49483-622)