Label: UMECTA MOUSSE UREA- urea foam aerosol, foam
-
Contains inactivated NDC Code(s)
NDC Code(s): 68712-020-01 - Packager: Innocutis Holdings LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated December 23, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Description
Rx only
For topical use only
Not for ophthalmic use
Umecta is a keratolytic, emollient which is a gentle, yet potent, tissue softener for nails and skin Each gram of Umecta mousse contains urea (40%), Butane, Butyrospermum Parkii (Shea Butter) Extract, Carbomer, Glycine Soya (Soy Bean) Sterol, Helianthus Annuus (Sunflower) Oil, Isobutane, Laureth-4, Polysorbate-20, Propane, Purified Water, Stearic Acid, Triethanolamine. Urea is a diamide of carbonic acid with the following chemical structure:
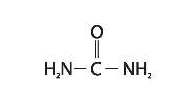
-
Clinical Pharmacology
Urea gently dissolves the intercellular matrix which results in loosening the horny layer of skin and shedding scaly skin at regular intervals, thereby softening hyperkeratotic areas. Urea also hydrates and gently dissolves the intercellular matrix of the nail plate which can result in the softening and eventual debridement of the nail plate.
- Pharmacokinetics
-
Indications and Usage
For debridement and promotion of normal healing of hyperkeratotic surface lesions, particularly where healing is retarded by local infection, necrotic tissue, fibrinous or purulent debris or eschar. Urea is useful for the treatment of hyperkeratotic conditions such as dry, rough skin, dermatitis, psoriasis, xerosis, ichthyosis, eczema, keratosis, keratoderma, corns, and calluses, as well as damaged, ingrown and devitalized nails.
- Contraindications
- Warnings
- Precautions
- Pregnancy
- Nursing Monthers
- Adverse Reactions
- Dosage and Administration
- How Supplied
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
UMECTA MOUSSE UREA
urea foam aerosol, foamProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:68712-020 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength UREA (UNII: 8W8T17847W) (UREA - UNII:8W8T17847W) UREA 400 mg in 1 g Inactive Ingredients Ingredient Name Strength BUTANE (UNII: 6LV4FOR43R) SHEA BUTTER (UNII: K49155WL9Y) CARBOMER 934 (UNII: Z135WT9208) SOYBEAN OIL (UNII: 241ATL177A) SUNFLOWER OIL (UNII: 3W1JG795YI) ISOBUTANE (UNII: BXR49TP611) LAURETH-4 (UNII: 6HQ855798J) POLYSORBATE 20 (UNII: 7T1F30V5YH) PROPANE (UNII: T75W9911L6) WATER (UNII: 059QF0KO0R) STEARIC ACID (UNII: 4ELV7Z65AP) TROLAMINE (UNII: 9O3K93S3TK) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68712-020-01 113.4 g in 1 CAN Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 09/01/2007 Labeler - Innocutis Holdings LLC (071501252) Establishment Name Address ID/FEI Business Operations American Spraytech LLC 137135237 manufacture


