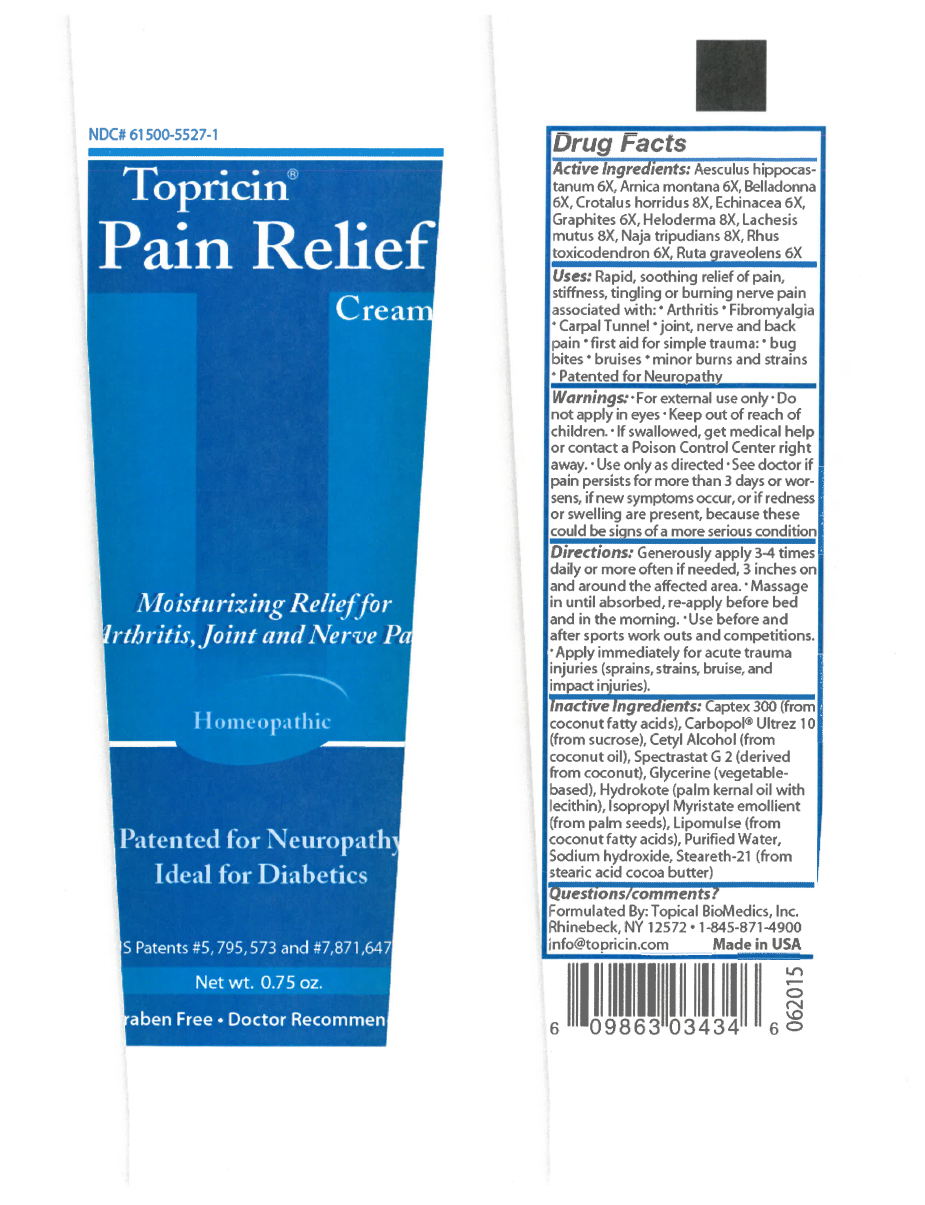
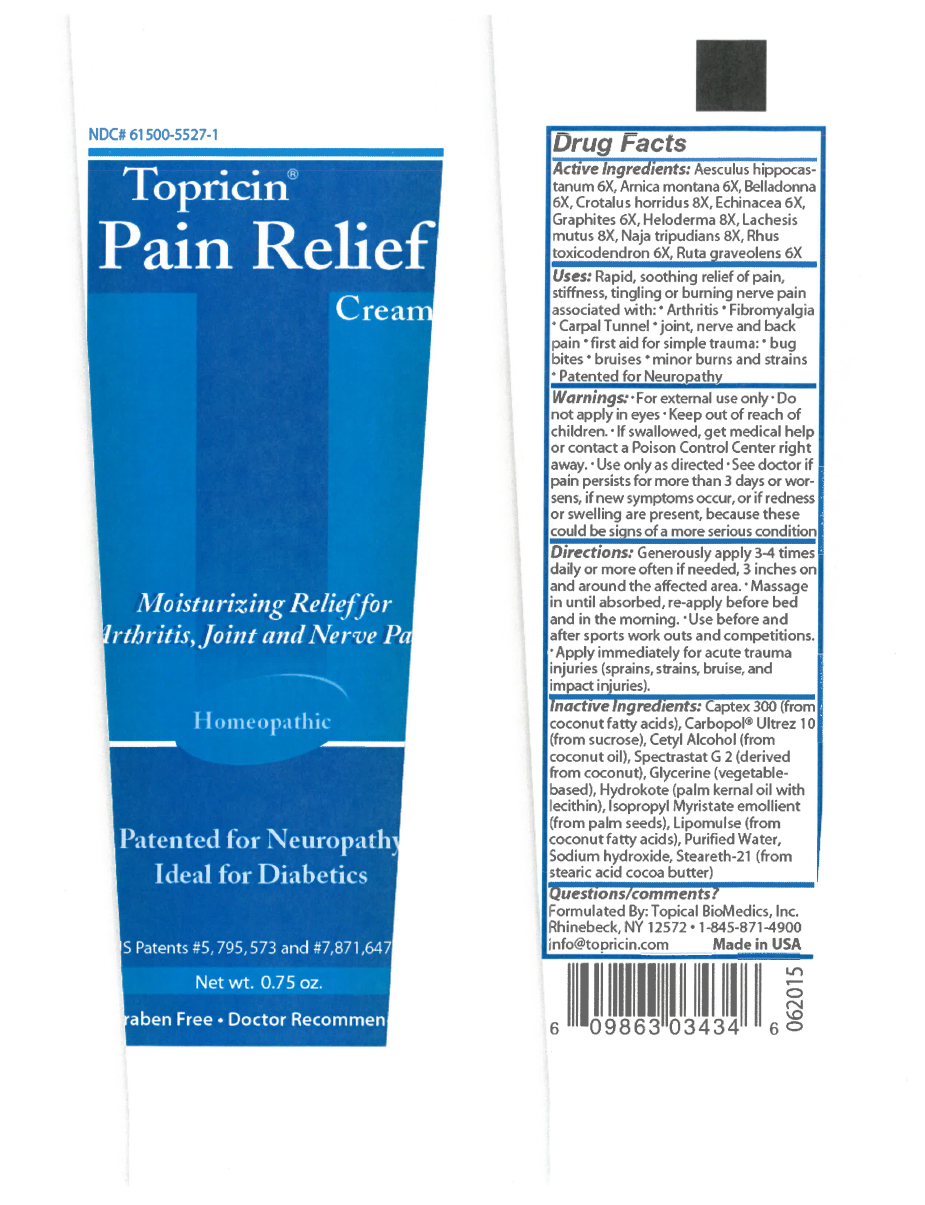
Label: TOPRICIN- aesculus hippocastanum, arnica montana, belladonna, crotalus horridus, echinacea, graphites, heloderma, lachesis mutus, naja tripudians, rhus toxicodendron, ruta graveolens cream
-
NDC Code(s):
61500-5527-1,
61500-5527-2,
61500-5527-3,
61500-5527-4, view more61500-5527-5, 61500-5527-6, 61500-5527-8
- Packager: Topical BioMedics, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated October 11, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
-
Uses
Rapid, soothing relief of pain, stiffness, tingling, or burning nerve pain associated with:
Arthritis and other joint pain and discomfort
Fibromyalgia
Carpal Tunnel Syndrome and other types of nerve pain
Pains & muscle cramps of the back, neck, shoulder, legs, hands & feet
Sports injuries (muscle pulls & soreness, impact, sprains, dislocations)
Repetitive motion injuries, such as bursitis, tendonitis and sciatica
First Aid for simple trauma (bruises, minor burns, cuts, scrapes & strains
Purpose
Relieves pain in the lower back, hip and spine
Treats pain of impact, falling injuries and contusion to muscles and joints
Treats muscle spasms night leg cramps
Relief of impact injuries & deep muscle bruising
Relieves sharp stitching pain in joints & muscles
Relieves skin conditions
Relief of burning pain in the hands and feet
Relief of sciatic pain and carpal tunnel
Relieves nerve injury pain
Pain relief of muscle cramping, joint and post-surgical pain
Relief of injuries to the knee, shin and elbow
-
Warnings
For external use only. Do not apply in eyes. Ask doctor or pharmacist before using if you are taking a presciption medication. Ask doctor or pharmacist before using if you are pregnant or nursing. Keep all medicines out of reach of children. Use only as directed, see doctor if pain persists for more than 3 days or worsens, if new symptoms occur, or if redness or swelling are present because these could be signs of a more serious condition. Before considering any self help regimen always consult your doctor, especially if you are taking prescription medication.
-
Directions
Generously apply 3-4 times daily or more often if needed, 3 inches on and around affected area. Massage in until absorbed, re-apply before bedtime and in the morning.
Use before and after sports work outs and other phys9ical acrivities.
Apply immediately for acute trauma injuries (sprain, strain, bruise, and impact injuries).
-
Inactive Ingredients
Inactive Ingredients:Captex 300 (from coconut fatty acids), Carbopol® Ultrez 10 (from sucrose), Cetyl Alcohol (from coconut oil), Spectrastat G 2 (derived from coconut), Glycerine (vegetable-based), Hydrokote (palm kernel oil with lecithin), Isopropyl Myristate emollient (from palm seeds), Lipomulse (from coconut fatty acids), Purified Water, Sodium hydroxide, Steareth-21 (from stearic acid cocoa butter)
-
Other Information
This homeopathic medicine is manufactured in the United States at the highest quality standards. Homeopathic medicines are safe, gentle and help your body heal. Safe for children (2-years and above), adults and the elderly. No odor or fragrance, no petroleum, no mineral oil, no lanolin, no capsaicin, no menthol, non-greasy, non-staining and paraben free. Store at room temperature. Tamper Eviden. Do not use if bottle has been opened.For severe injury, always seek medical attention.
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
TOPRICIN
aesculus hippocastanum, arnica montana, belladonna, crotalus horridus, echinacea, graphites, heloderma, lachesis mutus, naja tripudians, rhus toxicodendron, ruta graveolens creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:61500-5527 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AESCULUS HIPPOCASTANUM FLOWER (UNII: KK0Z92II8M) (AESCULUS HIPPOCASTANUM FLOWER - UNII:KK0Z92II8M) AESCULUS HIPPOCASTANUM FLOWER 6 [hp_X] in 170 g ARNICA MONTANA (UNII: O80TY208ZW) (ARNICA MONTANA - UNII:O80TY208ZW) ARNICA MONTANA 6 [hp_X] in 170 g CROTALUS HORRIDUS HORRIDUS VENOM (UNII: YHA2XLJ956) (CROTALUS HORRIDUS HORRIDUS VENOM - UNII:YHA2XLJ956) CROTALUS HORRIDUS HORRIDUS VENOM 8 [hp_X] in 170 g ECHINACEA ANGUSTIFOLIA (UNII: VB06AV5US8) (ECHINACEA ANGUSTIFOLIA - UNII:VB06AV5US8) ECHINACEA ANGUSTIFOLIA 6 [hp_X] in 170 g GRAPHITE (UNII: 4QQN74LH4O) (GRAPHITE - UNII:4QQN74LH4O) GRAPHITE 6 [hp_X] in 170 g HELODERMA HORRIDUM VENOM (UNII: O9M1UQ4YIO) (HELODERMA HORRIDUM VENOM - UNII:O9M1UQ4YIO) HELODERMA HORRIDUM VENOM 8 [hp_X] in 170 g LACHESIS MUTA VENOM (UNII: VSW71SS07I) (LACHESIS MUTA VENOM - UNII:VSW71SS07I) LACHESIS MUTA VENOM 8 [hp_X] in 170 g NAJA NAJA VENOM (UNII: ZZ4AG7L7VM) (NAJA NAJA VENOM - UNII:ZZ4AG7L7VM) NAJA NAJA VENOM 8 [hp_X] in 170 g RUTA GRAVEOLENS FLOWERING TOP (UNII: N94C2U587S) (RUTA GRAVEOLENS FLOWERING TOP - UNII:N94C2U587S) RUTA GRAVEOLENS FLOWERING TOP 6 [hp_X] in 170 g ATROPA BELLADONNA (UNII: WQZ3G9PF0H) (ATROPA BELLADONNA - UNII:WQZ3G9PF0H) ATROPA BELLADONNA 6 [hp_X] in 170 g TOXICODENDRON PUBESCENS LEAF (UNII: 6IO182RP7A) (TOXICODENDRON PUBESCENS LEAF - UNII:6IO182RP7A) TOXICODENDRON PUBESCENS LEAF 6 [hp_X] in 170 g Inactive Ingredients Ingredient Name Strength CETYL ALCOHOL (UNII: 936JST6JCN) CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) SODIUM HYDROXIDE (UNII: 55X04QC32I) CAPRYLHYDROXAMIC ACID (UNII: UPY805K99W) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) GLYCERIN (UNII: PDC6A3C0OX) PEG-100 STEARATE (UNII: YD01N1999R) WATER (UNII: 059QF0KO0R) STEARETH-21 (UNII: 53J3F32P58) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) HYDROGENATED PALM KERNEL OIL (UNII: FM8D1RE2VP) GLYCERYL CAPRYLATE (UNII: TM2TZD4G4A) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61500-5527-1 21 g in 1 TUBE; Type 0: Not a Combination Product 04/05/2016 2 NDC:61500-5527-5 170 g in 1 TUBE; Type 0: Not a Combination Product 04/01/2016 3 NDC:61500-5527-4 113 g in 1 JAR; Type 0: Not a Combination Product 04/01/2016 4 NDC:61500-5527-2 57 g in 1 TUBE; Type 0: Not a Combination Product 04/01/2016 5 NDC:61500-5527-8 227 g in 1 BOTTLE; Type 0: Not a Combination Product 04/01/2016 6 NDC:61500-5527-6 454 g in 1 BOTTLE; Type 0: Not a Combination Product 04/01/2016 7 NDC:61500-5527-3 907 g in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 04/01/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 04/01/2016 Labeler - Topical BioMedics, Inc. (125911037)