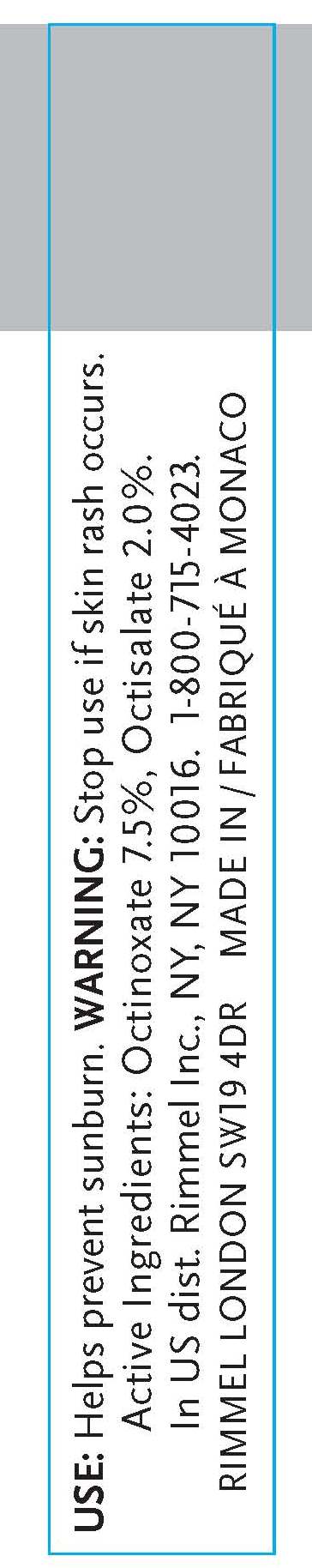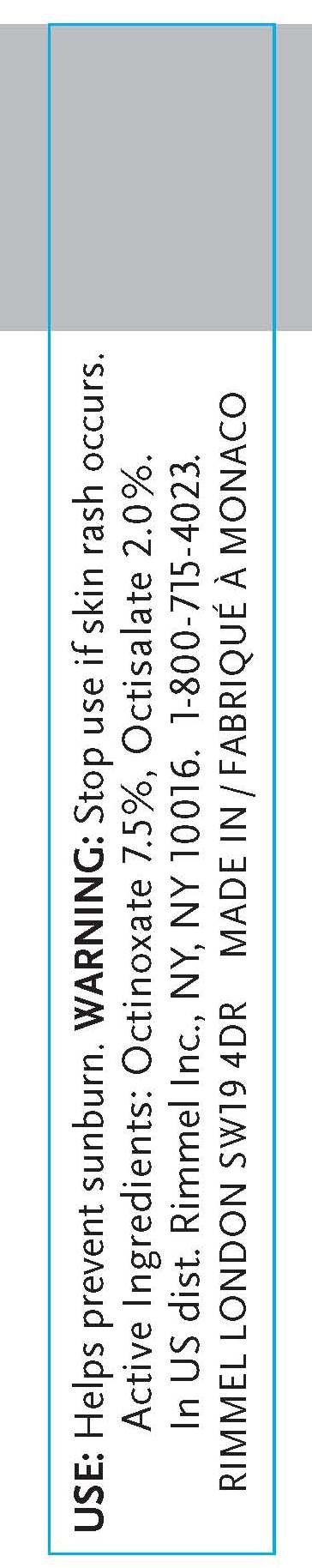Label: RIMMEL LONDON MOISTURE RENEW LIPGLOSS - FUSCIA THERAPHY (345)- octinoxate, octisalate cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 59351-0310-1 - Packager: Lancaster S.A.M.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated October 20, 2010
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- INDICATIONS & USAGE
- WARNINGS
- ACTIVE INGREDIENT
-
INACTIVE INGREDIENT
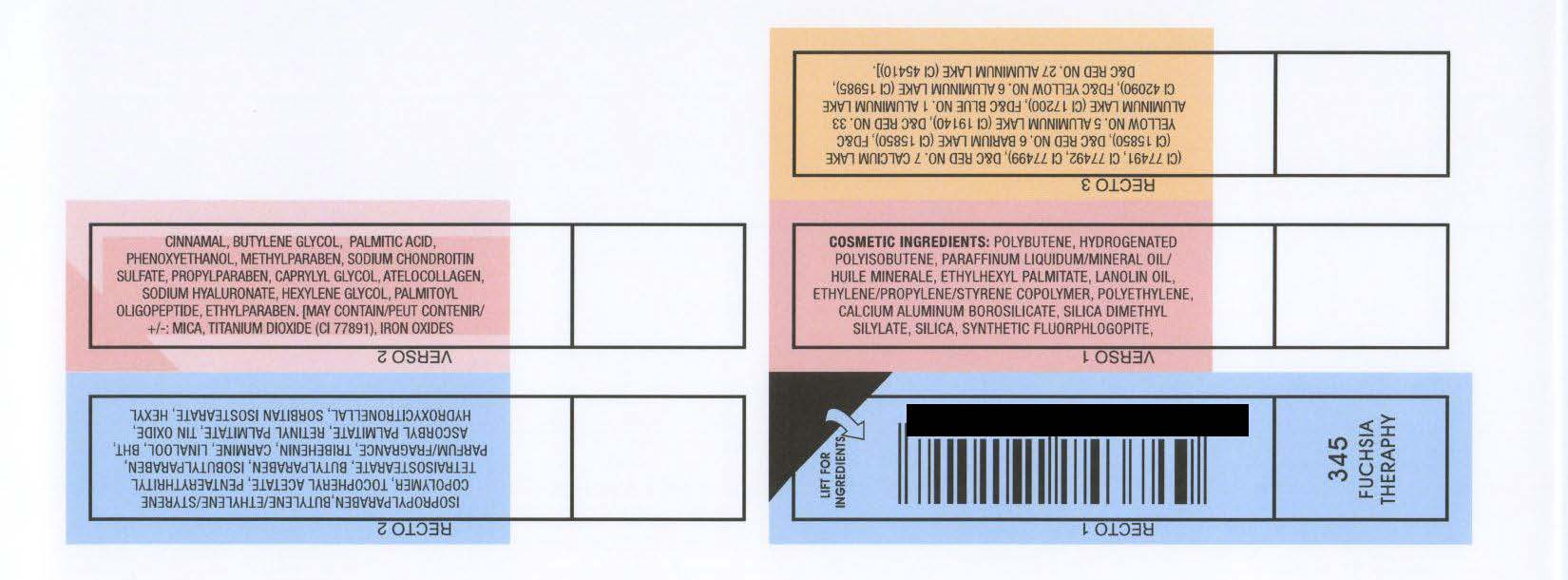
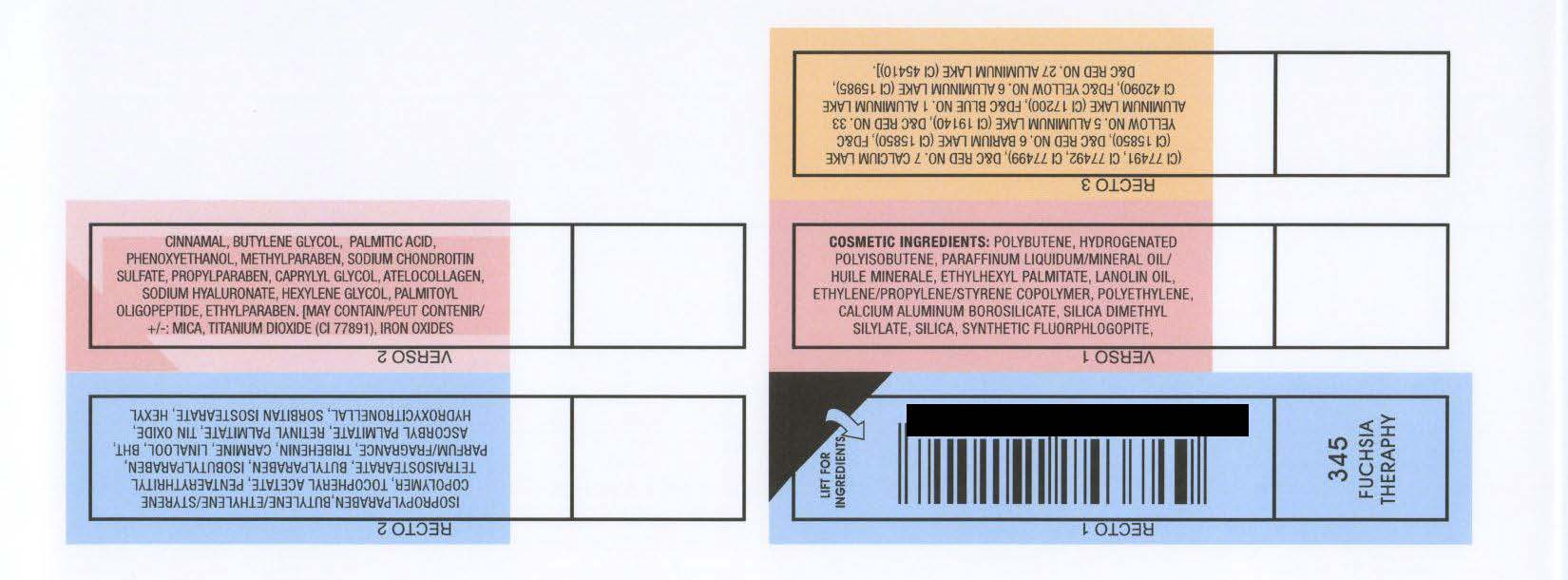
COSMETIC INGREDIENTS: POLYBUTENE, HYDROGENATED
POLYISOBUTENE, PARAFFINUM LIQUIDUM/MINERAL OIL/
HUILE MINERALE, ETHYLHEXYL PALMITATE, LANOLIN OIL,
ETHYLENE/PROPYLENE/STYRENE COPOLYMER, POLYETHYLENE,
CALCIUM ALUMINUM BOROSILICATE, SILICA DIMETHYL
SILYLATE, SILICA, SYNTHETIC FLUORPHLOGOPITE,
ISOPROPYLPARABEN,BUTYLENE/ETHYLENE/STYRENE
COPOLYMER, TOCOPHERYL ACETATE, PENTAERYTHRITYL
TETRAISOSTEARATE, BUTYLPARABEN, ISOBUTYLPARABEN,
PARFUM/FRAGRANCE, TRIBEHENIN, CARMINE, LINALOOL, BHT,
ASCORBYL PALMITATE, RETINYL PALMITATE, TIN OXIDE,
HYDROXYCITRONELLAL, SORBITAN ISOSTEARATE, HEXYL
CINNAMAL, BUTYLENE GLYCOL, PALMITIC ACID,
PHENOXYETHANOL, METHYLPARABEN, SODIUM CHONDROITIN
SULFATE, PROPYLPARABEN, CAPRYLYL GLYCOL, ATELOCOLLAGEN,
SODIUM HYALURONATE, HEXYLENE GLYCOL, PALMITOYL
OLIGOPEPTIDE, ETHYLPARABEN. [MAY CONTAIN/PEUT CONTENIR/
: MICA, TITANIUM DIOXIDE (CI 77891), IRON OXIDES
(CI 77491, CI 77492, CI 77499), DandC RED NO. 7 CALCIUM LAKE
(CI 15850), DandC RED NO. 6 BARIUM LAKE (CI 15850), FDandC
YELLOW NO. 5 ALUMINUM LAKE (CI 19140), DandC RED NO. 33
ALUMINUM LAKE (CI 17200), FDandC BLUE NO. 1 ALUMINUM LAKE
CI 42090), FDandC YELLOW NO. 6 ALUMINUM LAKE (CI 15985),
DandC RED NO. 27 ALUMINUM LAKE (CI 45410)].
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
RIMMEL LONDON MOISTURE RENEW LIPGLOSS - FUSCIA THERAPHY (345)
octinoxate, octisalate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59351-0310 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE .012 mL in 6 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE .12 mL in 6 mL Inactive Ingredients Ingredient Name Strength MINERAL OIL (UNII: T5L8T28FGP) ETHYLHEXYL PALMITATE (UNII: 2865993309) LANOLIN (UNII: 7EV65EAW6H) ETHYLENE (UNII: 91GW059KN7) ALUMINUM (UNII: CPD4NFA903) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ISOPROPYLPARABEN (UNII: A6EOX47QK0) ETHYLENE (UNII: 91GW059KN7) .ALPHA.-TOCOPHEROL ACETATE, D- (UNII: A7E6112E4N) BUTYLPARABEN (UNII: 3QPI1U3FV8) ISOBUTYLPARABEN (UNII: 0QQJ25X58G) TRIBEHENIN (UNII: 8OC9U7TQZ0) LINALOOL, (+/-)- (UNII: D81QY6I88E) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) ASCORBYL PALMITATE (UNII: QN83US2B0N) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) STANNIC OXIDE (UNII: KM7N50LOS6) HYDROXYCITRONELLAL (UNII: 8SQ0VA4YUR) SORBITAN (UNII: 6O92ICV9RU) .ALPHA.-HEXYLCINNAMALDEHYDE (UNII: 7X6O37OK2I) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) PALMITIC ACID (UNII: 2V16EO95H1) PHENOXYETHANOL (UNII: HIE492ZZ3T) METHYLPARABEN (UNII: A2I8C7HI9T) SULFATE ION (UNII: 7IS9N8KPMG) PROPYLPARABEN (UNII: Z8IX2SC1OH) CAPRYLYL GLYCOL (UNII: 00YIU5438U) HYALURONATE SODIUM (UNII: YSE9PPT4TH) HEXYLENE GLYCOL (UNII: KEH0A3F75J) ETHYLPARABEN (UNII: 14255EXE39) MICA (UNII: V8A1AW0880) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) IRON (UNII: E1UOL152H7) CALCIUM (UNII: SY7Q814VUP) BARIUM (UNII: 24GP945V5T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59351-0310-1 6 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 10/20/2010 Labeler - Lancaster S.A.M. (401011325) Registrant - Rimmel Inc. (965020402) Establishment Name Address ID/FEI Business Operations Lancaster S.A.M. 401011325 manufacture