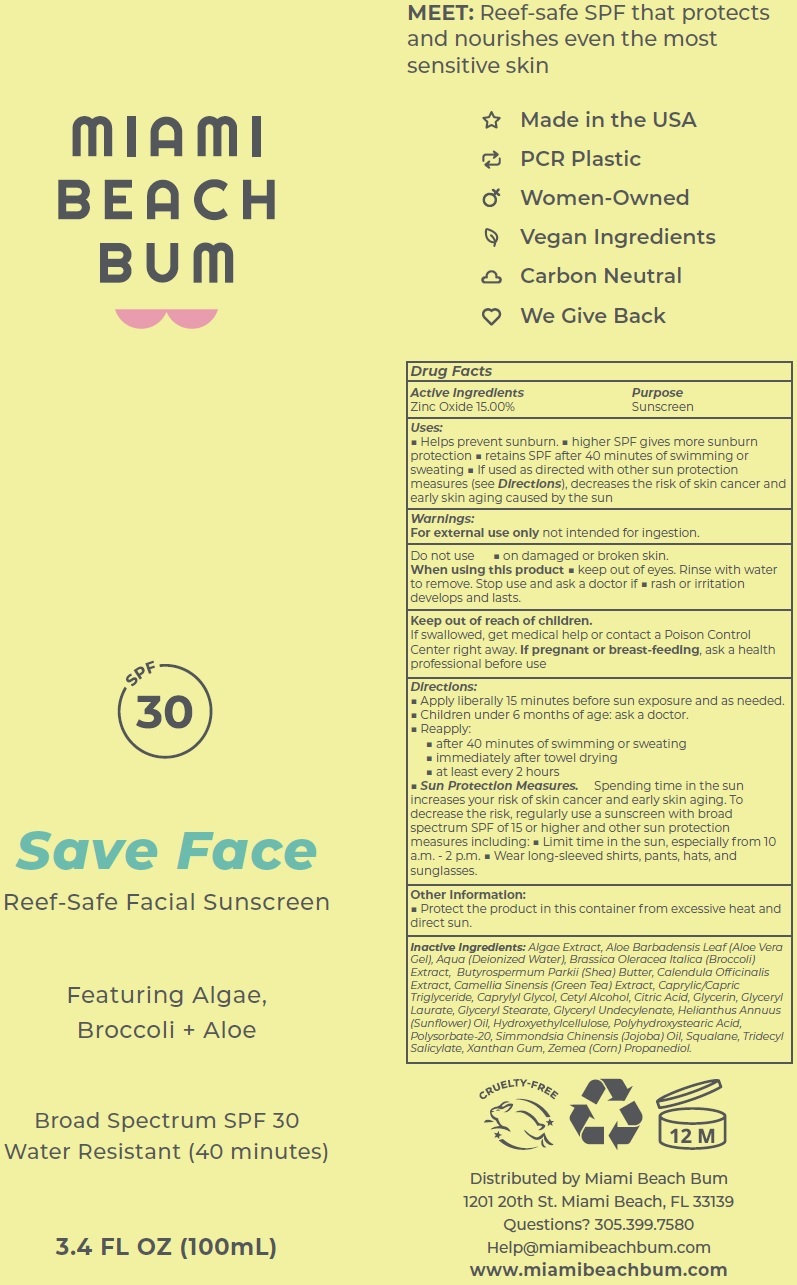
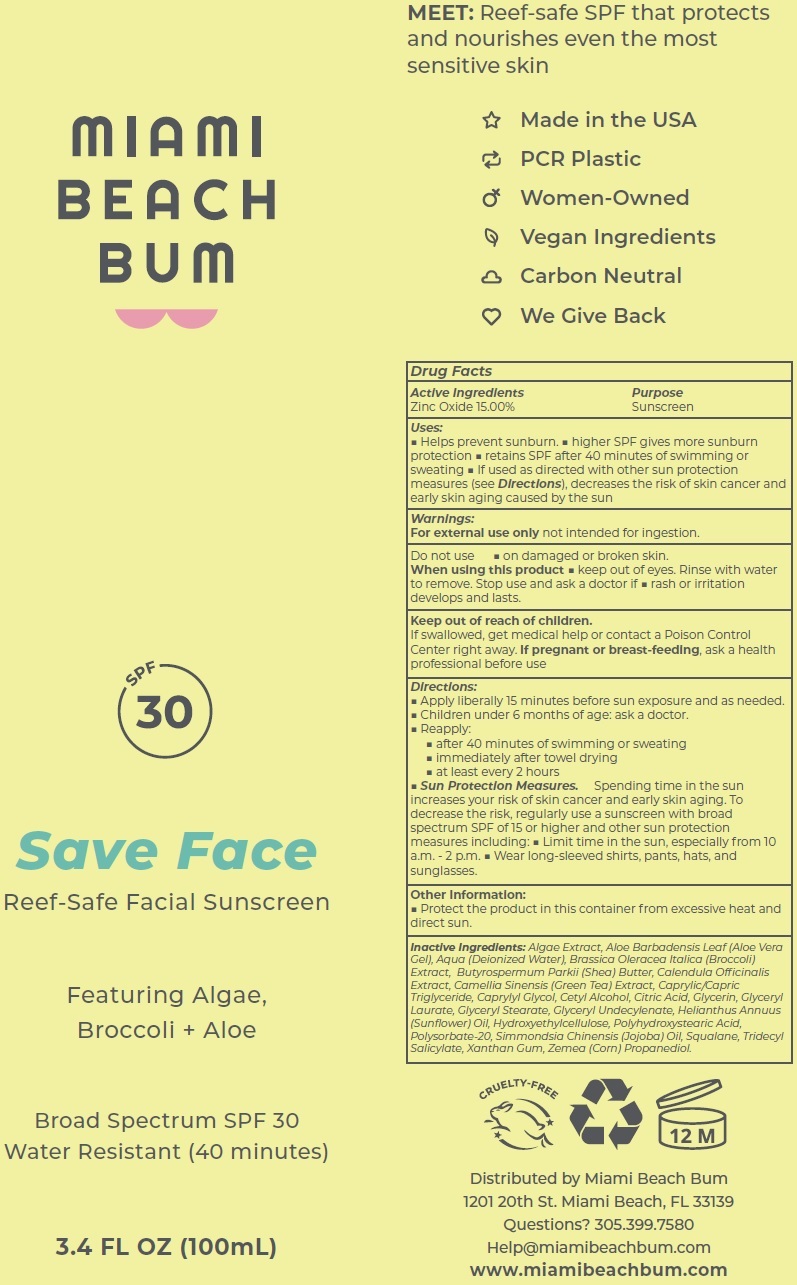
Label: MIAMI BEACH BUM SAVE FACE SPF-30 SUNSCREEN- zinc oxide cream
- NDC Code(s): 82812-336-00
- Packager: MIAMI BEACH BUM
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 10, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredients
- Uses:
- Warnings:
-
Directions:
- Apply liberally 15 minutes before sun exposure and as needed.
- Children under 6 months of age: ask a doctor.
- Reapply:
- after 40 minutes of swimming or sweating
- immediately after towel drying
- at least every 2 hours
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease the risk, regularly use a sunscreen with broad spectrum SPF of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10 a.m. - 2 p.m.
- Wear long-sleeved shirts, pants, hats, and sunglasses.
- Other Information:
-
Inactive Ingredients:
Algae Extract, Aloe Barbadensis Leaf (Aloe Vera Gel), Aqua (Deionized Water), Brassica Oleracea Italica (Broccoli) Extract, Butyrospermum Parkii (Shea) Butter, Calendula Officinalis Extract, Camellia Sinensis (Green Tea) Extract, Caprylic/Capric Triglyceride, Caprylyl Glycol, Cetyl Alcohol, Citric Acid, Glycerin, Glyceryl Laurate, Glyceryl Stearate, Glyceryl Undecylenate, Helianthus Annuus (Sunflower) Oil, Hydroxyethylcellulose, Polyhydroxystearic Acid, Polysorbate-20, Simmondsia Chinensis (Jojoba) Oil, Squalane, Tridecyl Salicylate, Xanthan Gum, Zemea (Corn) Propanediol.
- Questions?
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
MIAMI BEACH BUM SAVE FACE SPF-30 SUNSCREEN
zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82812-336 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 150 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) WATER (UNII: 059QF0KO0R) BROCCOLI (UNII: UOI4FT57BZ) SHEA BUTTER (UNII: K49155WL9Y) CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) GREEN TEA LEAF (UNII: W2ZU1RY8B0) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CETYL ALCOHOL (UNII: 936JST6JCN) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL LAURATE (UNII: Y98611C087) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) HELIANTHUS ANNUUS FLOWERING TOP (UNII: BKJ0J3D1BP) HYDROXYETHYL CELLULOSE, UNSPECIFIED (UNII: T4V6TWG28D) POLYSORBATE 20 (UNII: 7T1F30V5YH) JOJOBA OIL (UNII: 724GKU717M) SQUALANE (UNII: GW89575KF9) TRIDECYL SALICYLATE (UNII: AZQ08K38Z1) XANTHAN GUM (UNII: TTV12P4NEE) CORN (UNII: 0N8672707O) PROPANEDIOL (UNII: 5965N8W85T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82812-336-00 100 mL in 1 TUBE; Type 0: Not a Combination Product 07/05/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 07/05/2022 Labeler - MIAMI BEACH BUM (116222380)