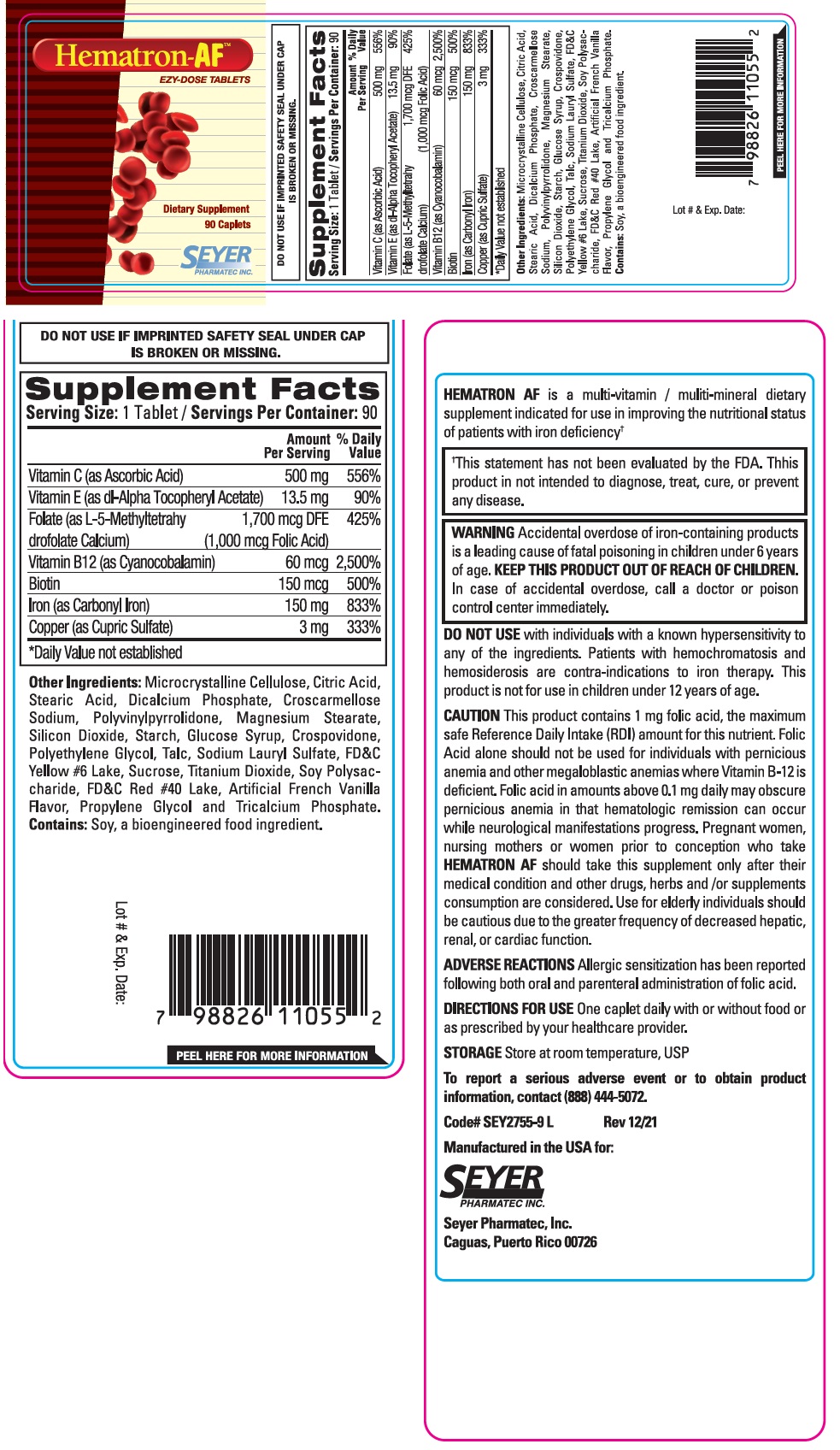
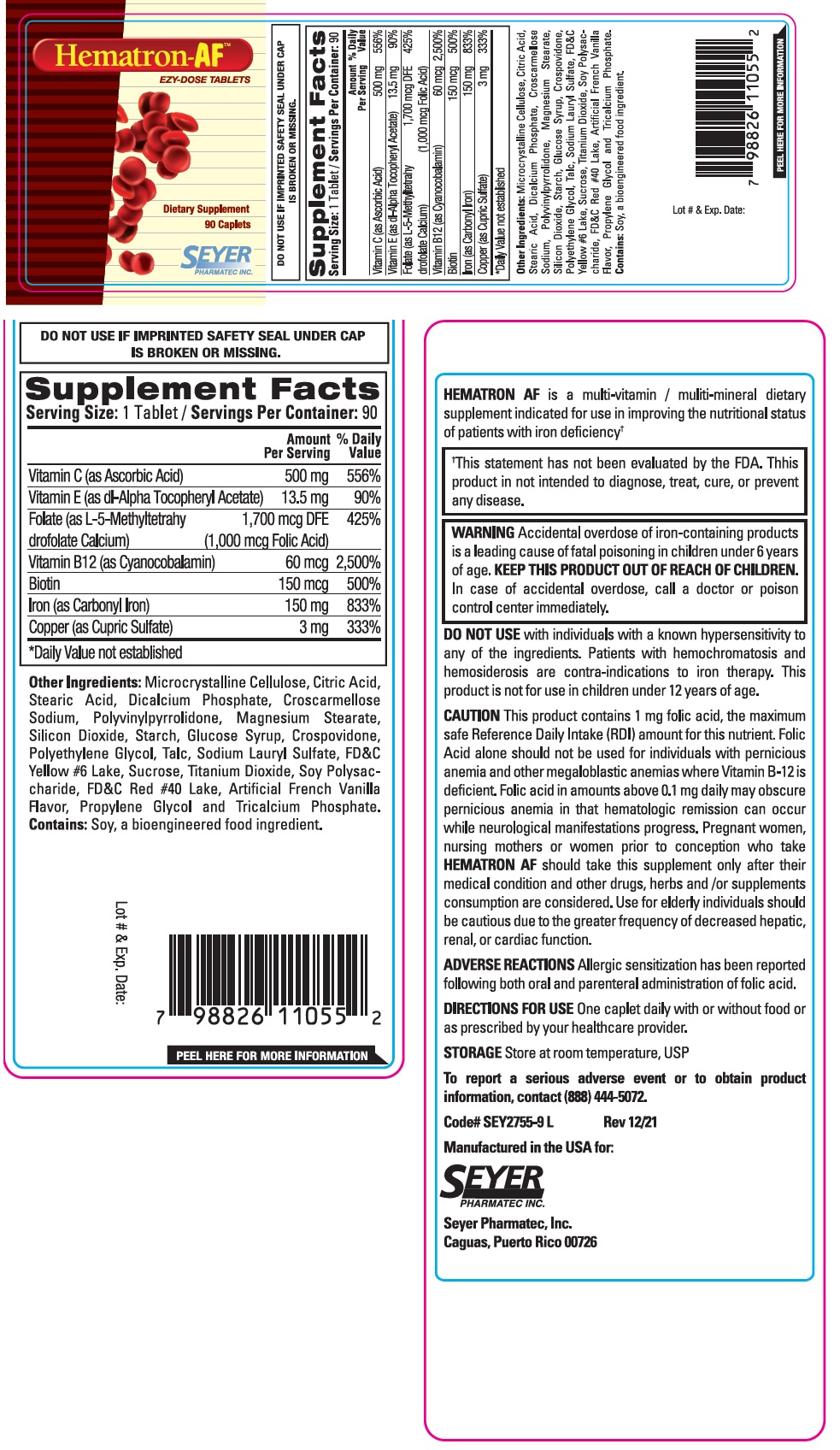
Label: HEMATRON-AF- ascorbic acid, dl-alpha tocopheryl acetate, l-5-methyltetrahydrofolate calcium, cyanocobalamin, biotin, carbonyl iron, cupric sulfate tablet
- NHRIC Code(s): 11026-2760-9
- Packager: Seyer Pharmatec, Inc.
- Category: DIETARY SUPPLEMENT
Drug Label Information
Updated February 15, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
STATEMENT OF IDENTITY
Supplement Facts Serving Size: 1 Tablet / Servings Per Container: 90 Amount % Daily Per Serving Value Vitamin C (as Ascorbic Acid) 500 mg 556% Vitamin E (as dl-Alpha Tocopheryl Acetate) 13.5 mg 90% Folate (as L-5-Methyltetrahydrofolate Calcium) 1,700 mcg DFE 425% (1,000 mcg Folic Acid) Vitamin B12 (as Cyanocobalamin) 60 mcg 2,500% Biotin 150 mcg 500% Iron (as Carbonyl Iron) 150 mg 833% Copper (as Cupric Sulfate) 3 mg 333% *Daily Value not established Other Ingredients:
Microcrystalline Cellulose, Citric Acid, Stearic Acid, Dicalcium Phosphate, Croscarmellose Sodium, Polyvinylpyrrolidone, Magnesium Stearate, Silicon Dioxide, Starch, Glucose Syrup, Crospovidone, Polyethylene Glycol, Talc, Sodium Lauryl Sulfate, FD&C Yellow #6 Lake, Sucrose, Titanium Dioxide, Soy Polysaccharide, FD&C Red #40 Lake, Artificial French Vanilla Flavor, Propylene Glycol and Tricalcium Phosphate.
Contains: Soy, a bioengineered food ingredient.HEMATRON AF is a multi-vitamin / multi-mineral dietary supplement indicated for use in improving the nutritional status of patients with iron deficiency†
†This statement has not been evaluated by the FDA. This product is not intended to diagnose, treat, cure, or prevent any disease. -
WARNINGS
WARNING Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6 years of age. KEEP THIS PRODUCT OUT OF REACH OF CHILDREN. In case of accidental overdose, call a doctor or poison control center immediately. DO NOT USE with individuals with a known hypersensitivity to any of the ingredients. Patients with hemochromatosis and hemosiderosis are contra-indications to iron therapy. This product is not for use in children under 12 years of age.
-
CAUTION
This product contains 1 mg folic acid, the maximum safe Reference Daily Intake (RDI) amount for this nutrient. Folic Acid alone should not be used for individuals with pernicious anemia and other megaloblastic anemias where Vitamin B-12 is deficient. Folic acid in amounts above 0.1 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurological manifestations progress. Pregnant Women, nursing mothers or women prior to conception who take HEMATRON AF should take this supplement only after their medical condition and other drugs, herbs and / or supplements consumption are considered. Use for elderly individuals should be cautious due to the greater frequency of decreased hepatic, renal, or cardiac function.
ADVERSE REACTIONS:
Allergic sensitization has been reported following both oral and parenteral administration of folic acid. - DIRECTIONS FOR USE
- STORAGE
- HEALTH CLAIM
- Packaging
-
INGREDIENTS AND APPEARANCE
HEMATRON-AF
ascorbic acid, dl-alpha tocopheryl acetate, l-5-methyltetrahydrofolate calcium, cyanocobalamin, biotin, carbonyl iron, cupric sulfate tabletProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:11026-2760 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 500 mg .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) (.ALPHA.-TOCOPHEROL, DL- - UNII:7QWA1RIO01) .ALPHA.-TOCOPHEROL, DL- 13.5 mg LEVOMEFOLATE CALCIUM (UNII: A9R10K3F2F) (LEVOMEFOLIC ACID - UNII:8S95DH25XC) LEVOMEFOLATE CALCIUM 1700 ug CYANOCOBALAMIN (UNII: P6YC3EG204) (CYANOCOBALAMIN - UNII:P6YC3EG204) CYANOCOBALAMIN 60 ug BIOTIN (UNII: 6SO6U10H04) (BIOTIN - UNII:6SO6U10H04) BIOTIN 150 ug IRON (UNII: E1UOL152H7) (IRON - UNII:E1UOL152H7) IRON 150 mg CUPRIC SULFATE (UNII: LRX7AJ16DT) (CUPRIC CATION - UNII:8CBV67279L) CUPRIC CATION 3 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) STEARIC ACID (UNII: 4ELV7Z65AP) ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) POVIDONE K60 (UNII: SZR7Z3Q2YH) MAGNESIUM STEARATE (UNII: 70097M6I30) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STARCH, CORN (UNII: O8232NY3SJ) CORN SYRUP (UNII: 9G5L16BK6N) CROSPOVIDONE, UNSPECIFIED (UNII: 2S7830E561) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) TALC (UNII: 7SEV7J4R1U) SODIUM LAURYL SULFATE (UNII: 368GB5141J) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) SUCROSE (UNII: C151H8M554) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) SOYBEAN COTYLEDON CELL WALL POLYSACCHARIDES (UNII: 4UL6DF56YQ) FD&C RED NO. 40 (UNII: WZB9127XOA) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) TRICALCIUM PHOSPHATE (UNII: K4C08XP666) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:11026-2760-9 90 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date dietary supplement 02/15/2023 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value color shape size (solid drugs) 21 mm scoring 1 imprint flavor Labeler - Seyer Pharmatec, Inc. (832947126)