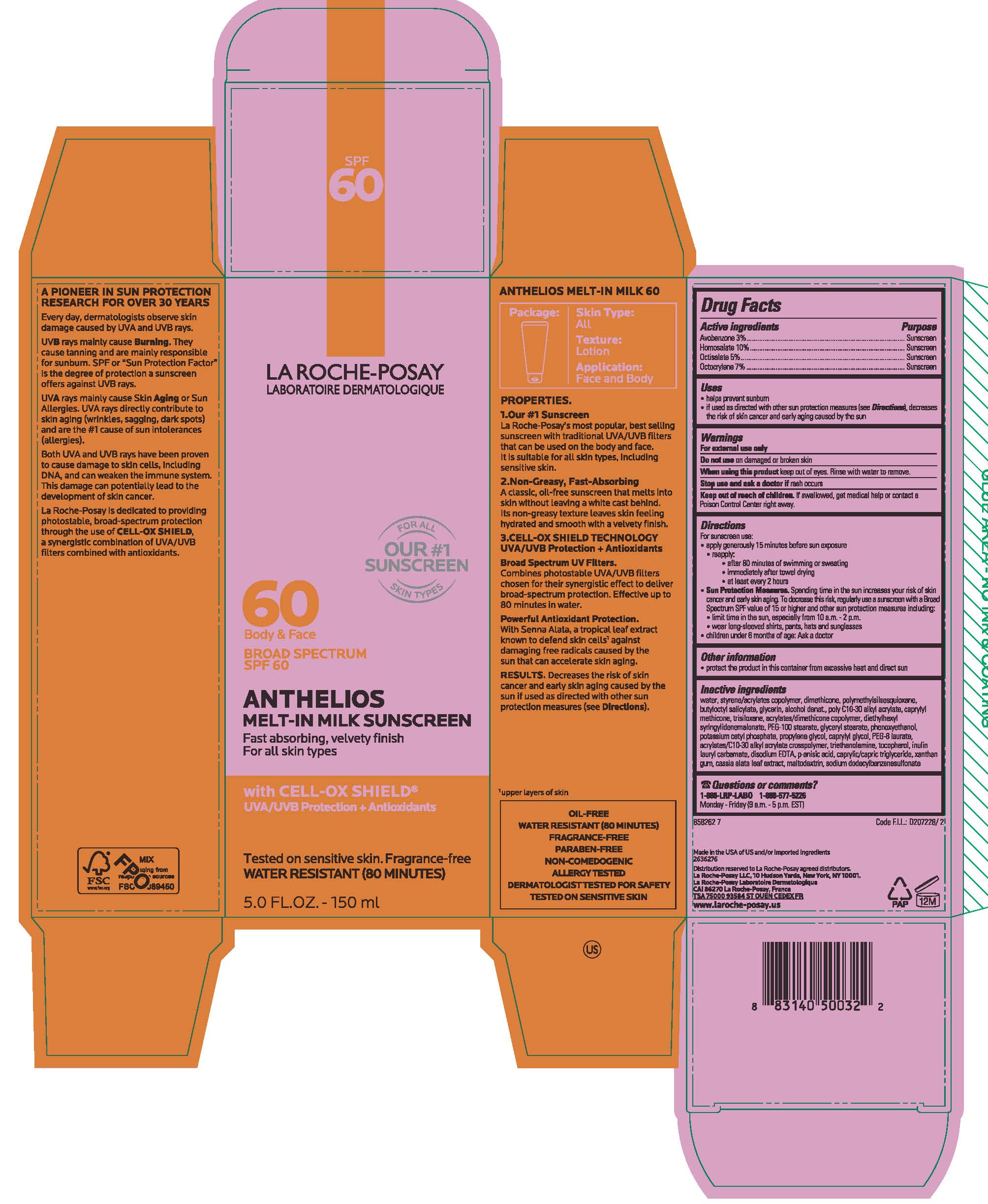
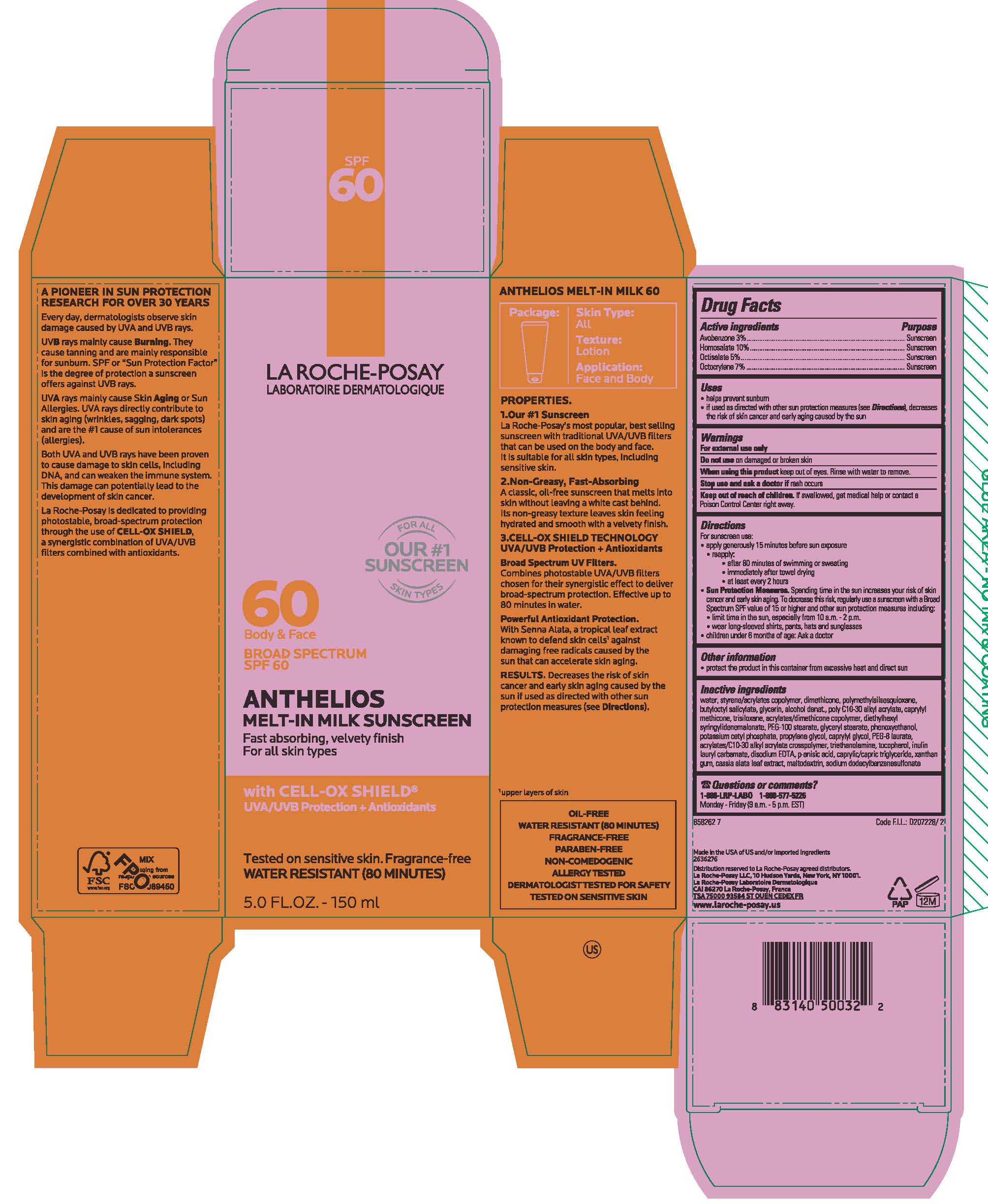
Label: LA ROCHE POSAY FACE BODY ANTHELIOS SPF 60 MELT IN MILK FAST ABSORBING, VELVETY FINISH FOR ALL SKIN TYPES WITH CELL-OC SHIELD XL- avobenzone, homosalate, octisalate and octocrylene lotion
-
NDC Code(s):
49967-355-01,
49967-355-02,
49967-355-03,
49967-355-04, view more49967-355-05, 49967-355-06
- Packager: L'Oreal USA Products Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 31, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
- Uses
- Warning
- Do not use
- When using this product
- Stop use and ask a doctor if
- Keep out of reach of children.
-
Directions
For sunscreen use:
• apply generously 15 minutes before sun exposure
• reapply:
• after 80 minutes of swimming or sweating
• immediately after towel drying
• at least every 2 hours
• Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
• limit time in the sun, especially from 10 a.m - 2 p.m.
• wear long-sleeved shirts, pants, hats and sunglasses
• children under 6 months of age: Ask a doctor - Other information
-
Inactive ingredients
water, sytrene/acrylates copolymer, dimethicone, polymethlysilsesquioxane, butylocytyl salicylate, glycerin, alcohol denat., poly C10-30 alkyl acrylate, caprylyl methicone, trisiloxane, acrylates/dimethicone copolymer, diethylhexyl syringylidenemalonate, PEG-100 stearate, glyceryl stearate, phenoxyethanol, potassium cetyl phosphate, propylene glycol, caprylyl glycol, PEG-8 laurate, acrylates/C10-30 alkyl acrylate crosspolymer, triethanolamine, tocopherol, inulin lauryl carbamate, disodium EDTA, p-anisic acid, caprylic/capric triglyceride, xanthan gum, cassia alata leaf extract, maltodextrin
- Questions or comments?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LA ROCHE POSAY FACE BODY ANTHELIOS SPF 60 MELT IN MILK FAST ABSORBING, VELVETY FINISH FOR ALL SKIN TYPES WITH CELL-OC SHIELD XL
avobenzone, homosalate, octisalate and octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49967-355 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 30 mg in 1 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 100 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 50 mg in 1 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 70 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIMETHICONE (UNII: 92RU3N3Y1O) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) GLYCERIN (UNII: PDC6A3C0OX) ALCOHOL (UNII: 3K9958V90M) CAPRYLYL TRISILOXANE (UNII: Q95M2P1KJL) TRISILANE (UNII: 1T3A75Z4ZL) DIETHYLHEXYL SYRINGYLIDENEMALONATE (UNII: 3V5U97P248) PEG-100 STEARATE (UNII: YD01N1999R) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PHENOXYETHANOL (UNII: HIE492ZZ3T) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CAPRYLYL GLYCOL (UNII: 00YIU5438U) PEG-8 LAURATE (UNII: 762O8IWA10) TROLAMINE (UNII: 9O3K93S3TK) TOCOPHEROL (UNII: R0ZB2556P8) EDETATE DISODIUM (UNII: 7FLD91C86K) P-ANISIC ACID (UNII: 4SB6Y7DMM3) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) XANTHAN GUM (UNII: TTV12P4NEE) SENNA ALATA LEAF (UNII: 4BXR6YZN92) MALTODEXTRIN (UNII: 7CVR7L4A2D) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49967-355-01 1 in 1 CARTON 12/15/2018 1 90 mL in 1 TUBE; Type 0: Not a Combination Product 2 NDC:49967-355-02 2 mL in 1 PACKET; Type 0: Not a Combination Product 04/01/2019 3 NDC:49967-355-03 1 in 1 CARTON 12/15/2018 3 50 mL in 1 TUBE; Type 0: Not a Combination Product 4 NDC:49967-355-04 1 in 1 CARTON 04/01/2019 4 150 mL in 1 TUBE; Type 0: Not a Combination Product 5 NDC:49967-355-05 15 mL in 1 TUBE; Type 0: Not a Combination Product 12/15/2018 6 NDC:49967-355-06 1000 mL in 1 BOTTLE; Type 0: Not a Combination Product 12/15/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 12/15/2018 Labeler - L'Oreal USA Products Inc (002136794) Establishment Name Address ID/FEI Business Operations L'OREAL USA, INC. 185931458 manufacture(49967-355) , pack(49967-355)