Label: ESKATA- hydrogen peroxide solution
-
Contains inactivated NDC Code(s)
NDC Code(s): 71180-001-01, 71180-001-03, 71180-001-12, 71180-002-01, view more71180-002-03, 71180-002-12 - Packager: Aclaris Therapeutics, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated March 27, 2019
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ESKATA® safely and effectively. See full prescribing information for ESKATA®.
ESKATA® (hydrogen peroxide) topical solution
Initial U.S. Approval: 2017INDICATIONS AND USAGE
ESKATA is indicated for the treatment of seborrheic keratoses that are raised. (1)
DOSAGE AND ADMINISTRATION
- To be administered by a healthcare provider. (2.1)
- For topical use only. Not for ophthalmic use. (2.1)
- Do not apply ESKATA topical solution to open or infected seborrheic keratoses. (2.1)
- Apply 4 times, approximately 1 minute apart, to the targeted lesion(s) during a single in-office treatment session. (2.1)
DOSAGE FORMS AND STRENGTHS
Topical solution: 40% (w/w) hydrogen peroxide (3)
CONTRAINDICATIONS
None (4)
WARNINGS AND PRECAUTIONS
- Eye Disorders: Avoid eye exposure. Eye disorders including corneal injury (erosion, ulceration, perforation, and scarring), chemical conjunctivitis, eyelid edema, severe eye pain, or permanent eye injury, including blindness can occur after exposure. If accidental exposure occurs, flush eyes with water for 15 to 30 minutes and initiate monitoring, and further evaluation as appropriate. (5.1)
- Local Skin Reactions: severe reactions, including ulcerations and scarring, may occur. Do not retreat with ESKATA until the skin has recovered from any reaction caused by the previous treatment (5.2)
ADVERSE REACTIONS
Common adverse reactions include erythema (99%), stinging (97%), edema (91%), scaling (90%), crusting (81%), and pruritus (58%). (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Aclaris Therapeutics, Inc at 1-833-225-2747 or FDA at 1-800-FDA-1088 or http://www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 3/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1. INDICATION AND USAGE
2. DOSAGE AND ADMINISTRATION
2.1 Important Administration Information
2.2 Dosage and Administration Instructions
3. DOSAGE FORMS AND STRENGTHS
4. CONTRAINDICATIONS
5. WARNINGS AND PRECAUTIONS
5.1 Eye Disorders
5.2 Local Skin Reactions
6. ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
8. USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10. OVERDOSE
11. DESCRIPTION
12. CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13. NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14. CLINICAL STUDIES
16. HOW SUPPLIED/STORAGE AND HANDLING
17. PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1. INDICATION AND USAGE
-
2. DOSAGE AND ADMINISTRATION
2.1 Important Administration Information
ESKATA is to be administered by a health care provider.
For topical use only. Not for oral, ophthalmic, or intravaginal use.
Do not apply ESKATA topical solution to open or infected seborrheic keratoses.
Prior to application of ESKATA, ensure the surface of seborrheic keratosis lesion is clear of oil and debris (an alcohol wipe can be used).
During a single in-office treatment session, apply ESKATA to seborrheic keratosis lesions 4 times, approximately 1 minute apart. After one use, replace the cap and discard the unit dose applicator.
When treating seborrheic keratoses on the face, take appropriate actions to ensure that ESKATA will not come into contact with the eyes.
If the treated lesions have not completely cleared approximately 3 weeks after treatment, another treatment may be administered following the same procedure.
2.2 Dosage and Administration Instructions
Preparation of the ESKATA applicator
Wear nitrile or vinyl examination gloves during the activation of the ESKATA applicator and during the administration of the solution to the lesion(s).
The method for preparing the ESKATA applicator for use is illustrated below. While activating the applicator, hold it away from the patient. Do not remove the cap until after completion of Step 4 (below).

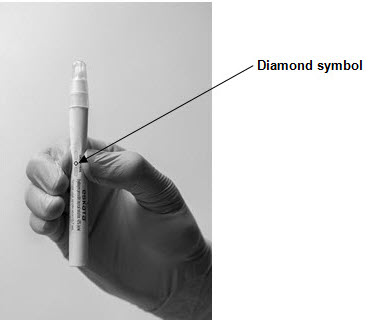
Step 1: Hold the ESKATA applicator so that the applicator cap is pointing up Step 2: Crush the ampule in the applicator by applying finger pressure to the diamond symbol on the applicator barrel 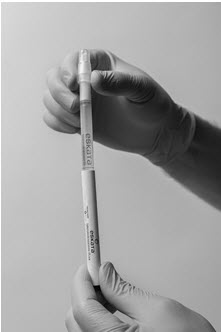
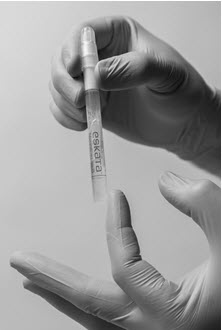
Step 3: Remove the sleeve Step 4: Holding the applicator with cap pointing up, tap the bottom of the applicator to separate the solution from the crushed ampule Application of ESKATA topical solution
Following release of the solution from the ampule, remove the cap from the ESKATA applicator. Gently squeeze the applicator barrel to express the solution to the applicator tip. Using the applicator, apply the solution directly to the seborrheic keratosis in a circular motion. Apply enough solution to uniformly wet the lesion surface, including the edges without excess running or dripping.
Wait 1 minute and observe. Whitening of the lesion may occur.
Do not progress to subsequent applications if severe erythema/edema or pain occur. Apply again in the same manner 3 additional applications 1 minute apart.
Minimize the amount of drug that comes into contact with surrounding skin. If ESKATA does come into contact with surrounding skin, use an absorbent wipe to remove any excess solution (do not use paper towels or tissue).
- 3. DOSAGE FORMS AND STRENGTHS
- 4. CONTRAINDICATIONS
-
5. WARNINGS AND PRECAUTIONS
5.1 Eye Disorders
Do not apply to the eyes or mucous membranes. Avoid treating seborrheic keratoses within the orbital rim. Direct contact with the eye can cause corneal injury (erosion, ulceration, perforation, and scarring), chemical conjunctivitis, eyelid edema, severe eye pain, or permanent eye injury, including blindness.
If accidental exposure occurs, flush with water for 15 to 30 minutes and initiate monitoring, and further evaluation as appropriate.
5.2 Local Skin Reactions
Skin reactions occurred in the treatment area after application of ESKATA. Severe local skin reactions included erosion, ulceration, vesiculation and scarring. [See Adverse Reactions (6.1)]. Do not initiate a second treatment course with ESKATA until the skin has recovered from any reaction caused by the previous treatment.
-
6. ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data described below reflect exposure to ESKATA or vehicle in a total of 937 subjects with seborrheic keratoses that are raised. Overall, 42% of the subjects were male and 58% were female. Ninety-eight (98) percent of the subjects were Caucasian and the mean age was 68.7 years.
At each visit, local skin reactions were graded for severity to determine the maximum severity after treatment. Table 1 presents the percentage of subjects with the local adverse reactions by the most severe grade reported during the course of the trials.
Table 1. Percentage of Subjects with Local Skin Reactions by Severity ESKATA
N=467Vehicle
N=470Mild Moderate Severe Total Mild Moderate Severe Total Erythema 13 67 19 99 29 5 <1 34 Stinging 34 49 15 97 9 1 <1 10 Edema 28 48 15 91 6 1 0 6 Scaling 49 36 5 90 28 5 1 33 Crusting 34 38 8 81 13 5 1 19 Pruritus 34 18 5 58 7 1 <1 8 Hyperpigmentation 32 7 <1 39 1 <1 0 1 Vesicles 21 3 1 24 <1 0 0 <1 Hypopigmentation 16 3 <1 19 1 <1 0 1 Erosion 12 2 1 15 <1 0 0 1 Ulceration 6 2 <1 9 1 1 0 2 Atrophy 4 0 0 4 0 0 0 0 Scarring 3 <1 <1 3 0 0 0 0 Common local skin reactions observed 10 minutes after treatment include: erythema (98%), stinging (93%), edema (85%), pruritus (32%), and vesiculation (18%).
Common local skin reactions observed 1 week after treatment are scaling (72%), erythema (66%), crusting (67%), pruritus (18%), erosion (9%), and ulceration (4%).
Common local skin reactions observed 15 weeks after the initial treatment are erythema (21%), hyperpigmentation (18%), scaling (16%), crusting (12%), and hypopigmentation (7%).
Less common adverse reactions occurring in ≥ 0.5% of subjects treated with ESKATA include eyelid edema (0.6%) and herpes zoster (0.6%).
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of ESKATA. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Skin and subcutaneous tissues disorders: crepitus
- 8. USE IN SPECIFIC POPULATIONS
- 10. OVERDOSE
-
11. DESCRIPTION
ESKATA (hydrogen peroxide) topical solution, 40% (w/w) is a clear, colorless solution for topical administration, which contains the active ingredient, hydrogen peroxide.
The chemical name of hydrogen peroxide is dihydrogen dioxide.
The molecular formula of hydrogen peroxide is H2O2 and the molecular weight is 34.01. Hydrogen peroxide is represented by the following structural formula:

ESKATA contains 40% (w/w) hydrogen peroxide in an aqueous solution of isopropyl alcohol and water.
-
12. CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism of action for ESKATA for the treatment of seborrheic keratosis is unknown.
12.2 Pharmacodynamics
The pharmacodynamics of ESKATA in the treatment of seborrheic keratosis are unknown.
12.3 Pharmacokinetics
Following application of ESKATA in patients with seborrheic keratosis lesions, hydrogen peroxide rapidly dissociates into water and reactive oxygen species. Indirect assessment of reactive oxygen species in patients with seborrheic keratosis lesions did not demonstrate any systemic absorption of hydrogen peroxide.
-
13. NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term animal studies have not been performed to evaluate the carcinogenic potential of ESKATA or hydrogen peroxide.
Hydrogen peroxide has been found to exhibit positive results in in vitro tests for genotoxicity, but has not exhibited positive results in in vivo tests for genotoxicity, presumably due to the rapid metabolism of hydrogen peroxide.
The effects of hydrogen peroxide on fertility have not been evaluated. Hydrogen peroxide has been associated with effects on sperm function and elevated testicular hydrogen peroxide concentration has been implicated in male infertility, although in vivo, no effect of hydrogen peroxide on sperm function has been demonstrated.
-
14. CLINICAL STUDIES
In two double-blind, vehicle-controlled clinical trials, 937 subjects with 4 clinically typical seborrheic keratoses that are raised on the face, trunk, or extremities were randomized to treatment with either ESKATA or vehicle. Subjects ranged from 42 to 91 years of age (mean 68.7 years), 58% percent were female, and 98% were Caucasian. A total of 925 subjects completed the trials. Each lesion was treated with 4 applications, at baseline and again at Day 22, if needed, and subjects were followed through Day 106.
Efficacy was assessed at Day 106. Success rate was defined as the proportion of subjects achieving "clear" on the Physician's Lesion Assessment Scale for all 4 treated lesions. Efficacy was also assessed for the proportion of subjects achieving "clear" on the Physician's Lesion Assessment Scale for at least 3 of 4 lesions. Table 3 presents the efficacy results for the two clinical trials.
Table 2. Percentage of Subjects Achieving Clearance of Target Lesions at Day 106 in Study 1 and Study 2 Study 1 Study 2 ESKATA
N=223Vehicle
N=227ESKATA
N=244Vehicle
N=243All 4 lesions "Clear" 4% 0% 8% 0% At least 3 of 4 lesions "Clear" 13% 0% 23% 0% -
16. HOW SUPPLIED/STORAGE AND HANDLING
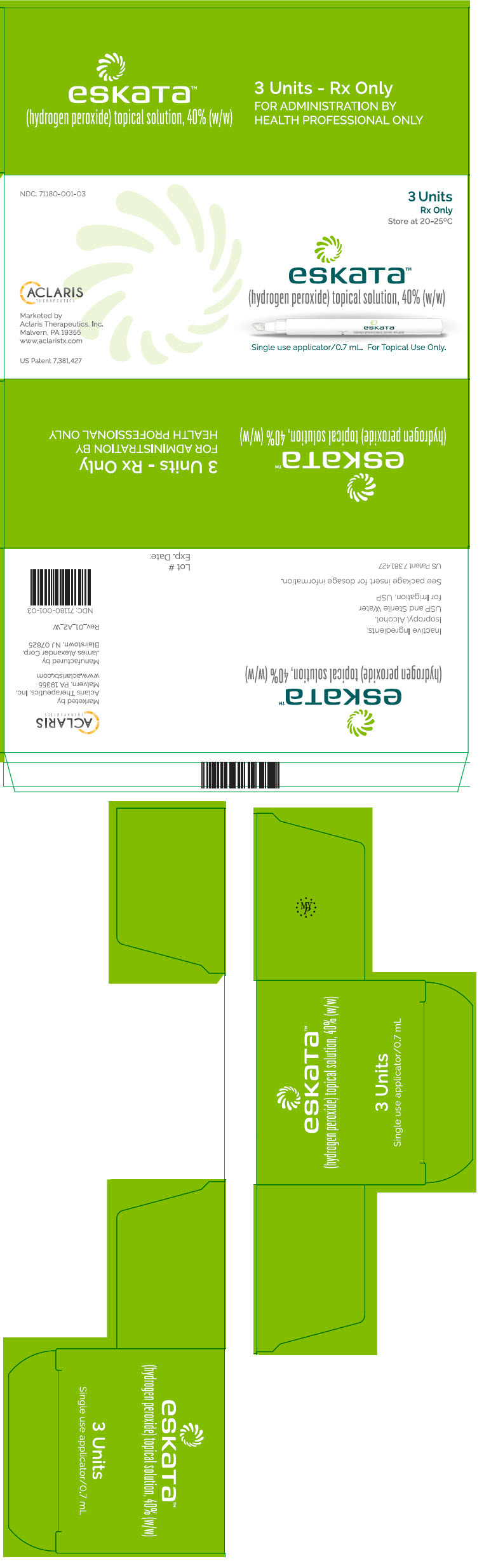
ESKATA (hydrogen peroxide) topical solution, 40% (w/w) is a clear, colorless solution and is supplied in a unit dose package. The available carton packages are presented below:
Dosage Strength Fill Volume Deliverable Volume Number of unit dose packages per carton NDC# 40% (w/w) 1.5 mL 0.7 mL 1 71180-001-01 3 71180-001-03 12 71180-001-12 2.2 mL 1.3 mL 1 71180-002-01 3 71180-002-03 12 71180-002-12 -
17. PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Ophthalmic Adverse Reactions
Inform patients that severe eye injury can occur with ESKATA application. Advise patients to inform the healthcare provider immediately if ESKATA runs into eyes, mouth, or nose during administration [see Warnings and Precautions (5.1)].
Local Skin Reactions
Inform patients that treatment with ESKATA may lead to local skin reactions [see Warnings and Precautions (5.2)].
- SPL UNCLASSIFIED SECTION
-
PATIENT PACKAGE INSERT
This Patient Information has been approved by the U.S. Food and Drug Administration Issued: December 2017 Patient Information
ESKATA® (es-KAH-tah)
(hydrogen peroxide), topical solutionIMPORTANT: ESKATA topical solution is for use as an in-office treatment. ESKATA is applied by your healthcare provider and is not for use at home. What is ESKATA ?
ESKATA is a prescription medicine used to treat seborrheic keratoses that are raised.Before treatment with ESKATA, tell your healthcare provider about all of your medical conditions, including if you: - are being treated or have had treatments for seborrheic keratosis
- have other skin problems
- are pregnant or plan to become pregnant.
- are breastfeeding or plan to breastfeed.
How should I receive ESKATA ? - Your healthcare provider will apply ESKATA to your seborrheic keratosis lesions.
- Your healthcare provider may apply ESKATA again, about 3 weeks after your treatment if your treated lesions are not completely gone.
What are the possible side effects with ESKATA?
ESKATA can cause serious side effects, including:- Eye problems. Eye problems can happen if ESKATA gets into your eyes, including:
- ulcers or small holes in your eyes
- scarring
- redness
- irritation
- eyelid swelling
- severe eye pain
- permanent eye injury, including blindness
If ESKATA accidentally gets into your eyes, your healthcare provider will tell you to flush them well with water for 15 to 30 minutes. Your healthcare provider may send you to another healthcare care provider if needed. - Local skin reactions. Skin reactions have happened in and around the treatment area after application of ESKATA. Severe skin reactions can include: breakdown of the outer layer of the skin (erosion), ulcers, blisters and scarring. Tell your healthcare provider if you have any skin reactions during treatment with ESKATA.
Your healthcare provider will not apply another treatment of ESKATA if your treated area is still irritated from the previous treatment.
Tell your healthcare provider right away if ESKATA gets into your eyes, mouth or nose during application.
These are not all of the possible side effects of ESKATA.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.General information about the safe and effective use of ESKATA.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. You can ask your pharmacist or healthcare provider for information that is written for healthcare professionals.What are the ingredients in ESKATA?
Active ingredient: hydrogen peroxide
Inactive ingredients: isopropyl alcohol and water.
Manufactured & Packaged by: James Alexander Corp., 845 Route 94, Blairstown, NJ 07825 United States
For: Aclaris Therapeutics, Inc., 640 Lee Rd, Suite 200, Wayne, PA 19087 United States
US Patent Number: US 7,381,427, US 9,675,639, US 9,980,983 and US 10,098,910 - PRINCIPAL DISPLAY PANEL - 0.7 mL Applicator Carton
- PRINCIPAL DISPLAY PANEL - 1.3 mL Applicator Carton
-
INGREDIENTS AND APPEARANCE
ESKATA
hydrogen peroxide solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:71180-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROGEN PEROXIDE (UNII: BBX060AN9V) (HYDROGEN PEROXIDE - UNII:BBX060AN9V) HYDROGEN PEROXIDE 40 mg in 100 mg Inactive Ingredients Ingredient Name Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71180-001-01 1 in 1 CARTON 12/14/2017 1 280 mg in 1 APPLICATOR; Type 0: Not a Combination Product 2 NDC:71180-001-03 3 in 1 CARTON 12/14/2017 2 280 mg in 1 APPLICATOR; Type 0: Not a Combination Product 3 NDC:71180-001-12 12 in 1 CARTON 12/14/2017 3 280 mg in 1 APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA209305 12/14/2017 ESKATA
hydrogen peroxide solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:71180-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROGEN PEROXIDE (UNII: BBX060AN9V) (HYDROGEN PEROXIDE - UNII:BBX060AN9V) HYDROGEN PEROXIDE 40 mg in 100 mg Inactive Ingredients Ingredient Name Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71180-002-01 1 in 1 CARTON 12/14/2017 1 520 mg in 1 APPLICATOR; Type 0: Not a Combination Product 2 NDC:71180-002-03 3 in 1 CARTON 12/14/2017 2 520 mg in 1 APPLICATOR; Type 0: Not a Combination Product 3 NDC:71180-002-12 12 in 1 CARTON 12/14/2017 3 520 mg in 1 APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA209305 12/14/2017 Labeler - Aclaris Therapeutics, Inc. (032365882) Establishment Name Address ID/FEI Business Operations James Alexander Corp. 040756421 ANALYSIS(71180-001, 71180-002) , LABEL(71180-001, 71180-002) , MANUFACTURE(71180-001, 71180-002)