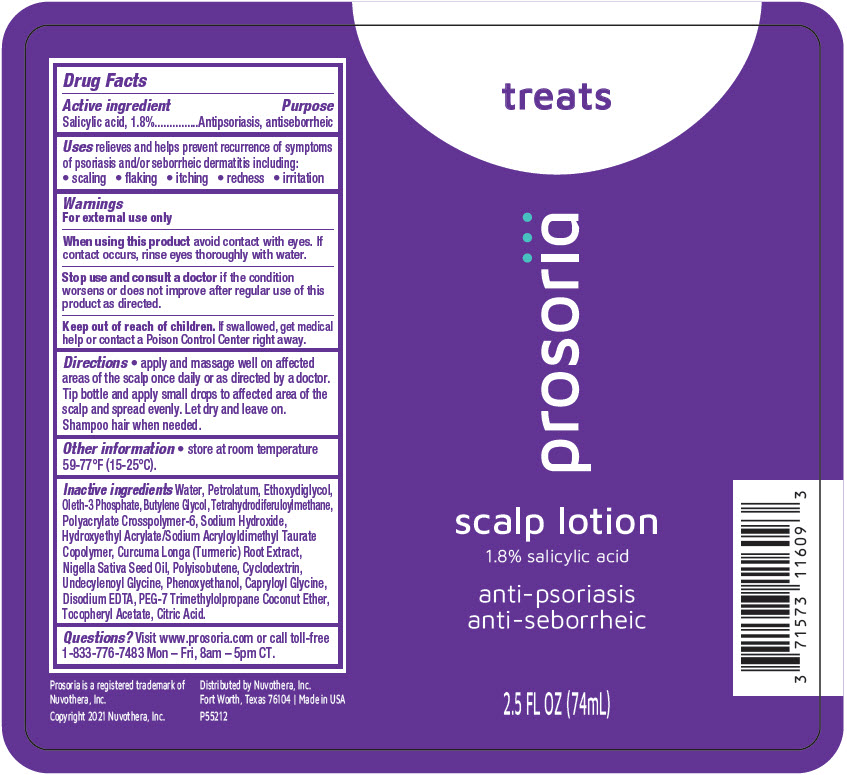
Label: PROSORIA SCALP- salicylic acid lotion/shampoo
- NDC Code(s): 71573-116-09
- Packager: Nuvothera, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 10, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
- Other information
-
Inactive ingredients
Water, Petrolatum, Ethoxydiglycol, Oleth-3 Phosphate, Butylene Glycol, Tetrahydrodiferuloylmethane, Polyacrylate Crosspolymer-6, Sodium Hydroxide, Hydroxyethyl Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Curcuma Longa (Turmeric) Root Extract, Nigella Sativa Seed Oil, Polyisobutene, Cyclodextrin, Undecylenoyl Glycine, Phenoxyethanol, Capryloyl Glycine, Disodium EDTA, PEG-7 Trimethylolpropane Coconut Ether, Tocopheryl Acetate, Citric Acid.
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 74 mL Bottle Label
-
INGREDIENTS AND APPEARANCE
PROSORIA SCALP
salicylic acid lotion/shampooProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71573-116 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Salicylic Acid (UNII: O414PZ4LPZ) (Salicylic Acid - UNII:O414PZ4LPZ) Salicylic Acid 1.33 g in 74 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Diethylene Glycol Monoethyl Ether (UNII: A1A1I8X02B) AMMONIUM ACRYLOYLDIMETHYLTAURATE, DIMETHYLACRYLAMIDE, LAURYL METHACRYLATE AND LAURETH-4 METHACRYLATE COPOLYMER, TRIMETHYLOLPROPANE TRIACRYLATE CROSSLINKED (45000 MPA.S) (UNII: Q7UI015FF9) Tetrahydrodiferuloylmethane (UNII: 00U0645U03) TURMERIC (UNII: 856YO1Z64F) Petrolatum (UNII: 4T6H12BN9U) Oleth-3 Phosphate (UNII: 8Q0Z18J1VL) Edetate Disodium Anhydrous (UNII: 8NLQ36F6MM) Sodium Hydroxide (UNII: 55X04QC32I) Butylene Glycol (UNII: 3XUS85K0RA) Nigella Sativa Seed Oil (UNII: CS4U38E731) Citric Acid Monohydrate (UNII: 2968PHW8QP) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) POLYISOBUTYLENE (1300 MW) (UNII: 241BN7J12Y) BETADEX (UNII: JV039JZZ3A) Phenoxyethanol (UNII: HIE492ZZ3T) HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER (100000 MPA.S AT 1.5%) (UNII: 86FQE96TZ4) Undecylenoyl Glycine (UNII: 4D20464K2J) Capryloyl Glycine (UNII: 8TY5YO42NJ) PEG-7 Trimethylolpropane Coconut Ether (UNII: MVJ3AD73GG) Product Characteristics Color YELLOW (Off White Light Yellow) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71573-116-09 74 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 04/01/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M032 04/01/2022 Labeler - Nuvothera, Inc. (080499864) Establishment Name Address ID/FEI Business Operations Swiss-American CDMO 080170933 MANUFACTURE(71573-116)