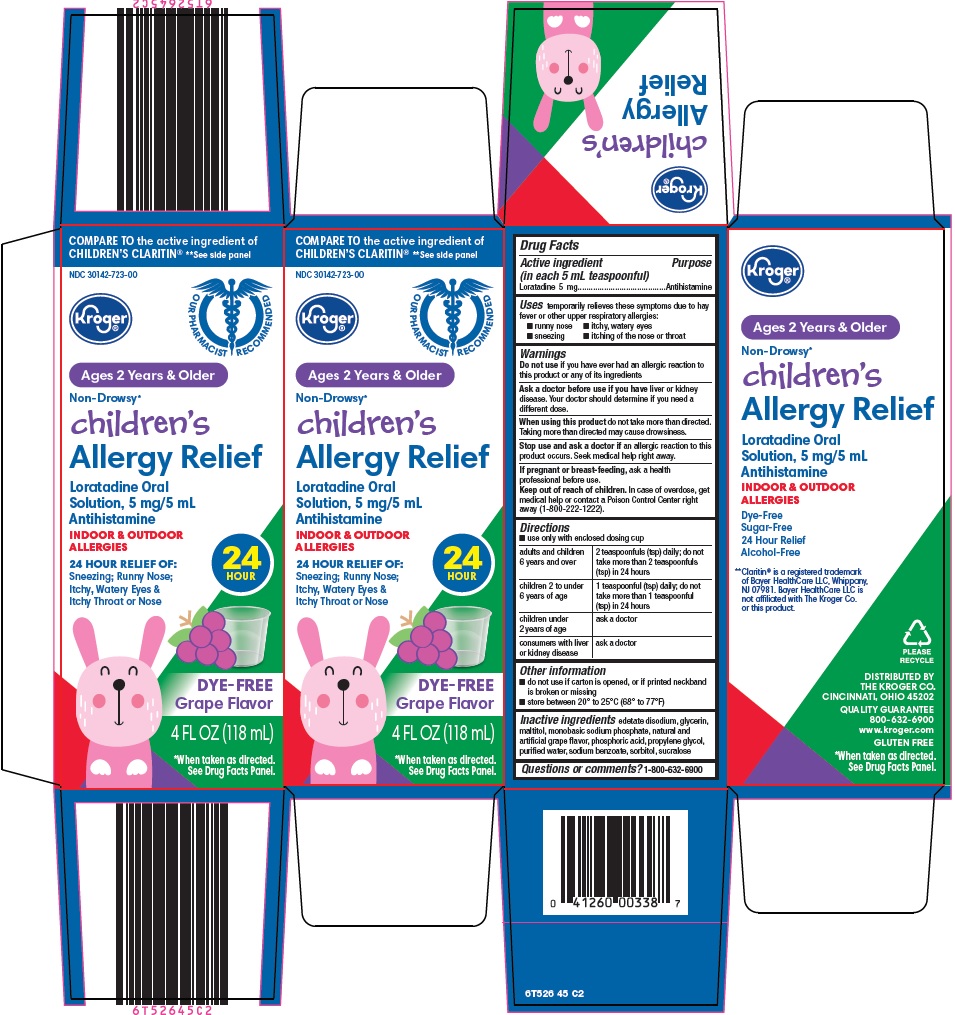
Label: CHILDRENS ALLERGY RELIEF- loratadine solution
- NDC Code(s): 30142-723-00, 30142-723-34
- Packager: Kroger Company
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated March 2, 2022
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each 5 mL teaspoonful)
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product
do not take more than directed. Taking more than directed may cause drowsiness.
-
Directions
- •
- use only with enclosed dosing cup
adults and children 6 years and over
2 teaspoonfuls (tsp) daily; do not take more than 2 teaspoonfuls (tsp) in 24 hours
children 2 to under 6 years of age
1 teaspoonful (tsp) daily; do not take more than 1 teaspoonful (tsp) in 24 hours
children under 2 years of age
ask a doctor
consumers with liver or kidney disease
ask a doctor
- Other information
- Inactive ingredients
- Questions or comments?
-
Package/Label Principal Display Panel
COMPARE TO the active ingredient of CHILDREN’S CLARITIN® See side panel
OUR PHARMACIST RECOMMENDED
Ages 2 Years & Older
Non-Drowsy*
children’s Allergy Relief
Loratadine Oral Solution, 5 mg/5 mL
Antihistamine
INDOORS & OUTDOOR ALLERGIES
24 HOUR
24 HOUR RELIEF OF:
Sneezing; Runny Nose; Itchy, Watery Eyes & Itchy Throat or Nose
DYE-FREE
Grape Flavor
4 FL OZ (118 mL)
*When taken as directed.
See Drug Facts Panel.
-
INGREDIENTS AND APPEARANCE
CHILDRENS ALLERGY RELIEF
loratadine solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:30142-723 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LORATADINE (UNII: 7AJO3BO7QN) (LORATADINE - UNII:7AJO3BO7QN) LORATADINE 5 mg in 5 mL Inactive Ingredients Ingredient Name Strength EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERIN (UNII: PDC6A3C0OX) MALTITOL (UNII: D65DG142WK) SODIUM PHOSPHATE, MONOBASIC, UNSPECIFIED FORM (UNII: 3980JIH2SW) PHOSPHORIC ACID (UNII: E4GA8884NN) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SORBITOL (UNII: 506T60A25R) SUCRALOSE (UNII: 96K6UQ3ZD4) Product Characteristics Color Score Shape Size Flavor GRAPE Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:30142-723-00 1 in 1 CARTON 07/30/2020 1 118 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:30142-723-34 1 in 1 CARTON 03/02/2022 2 240 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA075728 07/30/2020 Labeler - Kroger Company (006999528)