Label: CHEST CONGESTION RELIEF DM- dextromethorphan hydrobromide, guaifenesin liquid
- NDC Code(s): 0904-7134-70
- Packager: Major Pharmaceuticals
- This is a repackaged label.
- Source NDC Code(s): 0536-1313
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 13, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Warnings
Do not use if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease) or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Do not use if you have ever had an allergic reaction to any of the ingredients in this product.
Ask a doctor before use if you have
- •
- a cough with too much phlegm (mucus)
- •
- a cough that lasts or is chronic such as occurs with smoking asthma, chronic bronchitis or emphysema.
Stop use and ask a doctor if cough lasts more than 7 days, comes back or is accompanied by fever, rash, or headache that lasts. These could be signs of a serious condition.
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- INACTIVE INGREDIENT
- Other Information
- SPL UNCLASSIFIED SECTION
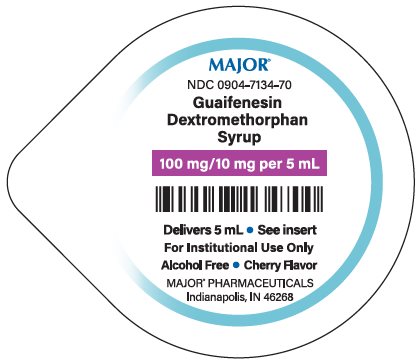
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CHEST CONGESTION RELIEF DM
dextromethorphan hydrobromide, guaifenesin liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0904-7134(NDC:0536-1313) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 10 mg in 5 mL GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 100 mg in 5 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) FD&C RED NO. 40 (UNII: WZB9127XOA) MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) water (UNII: 059QF0KO0R) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) SUCRALOSE (UNII: 96K6UQ3ZD4) SUCROSE (UNII: C151H8M554) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0904-7134-70 100 in 1 CASE 10/01/2020 1 5 mL in 1 CUP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 10/01/2020 Labeler - Major Pharmaceuticals (191427277)