Label: CHLORZOXAZONE tablet
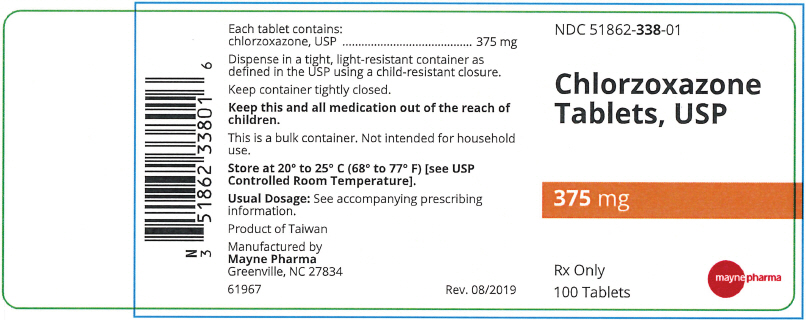
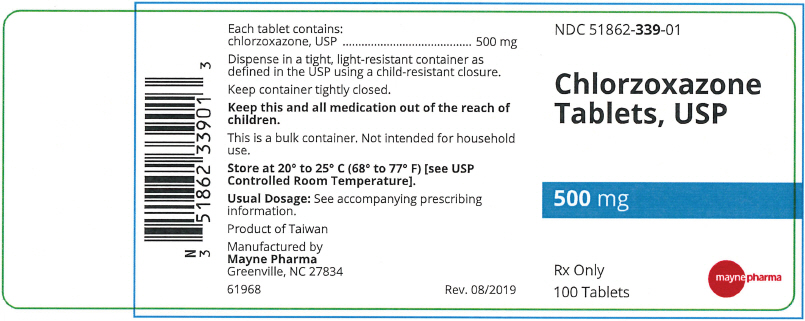
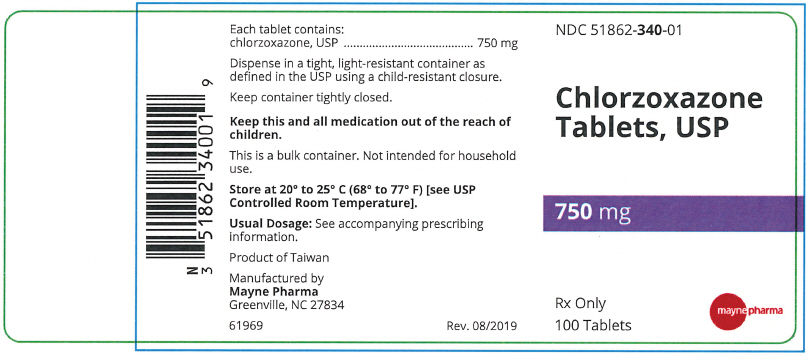
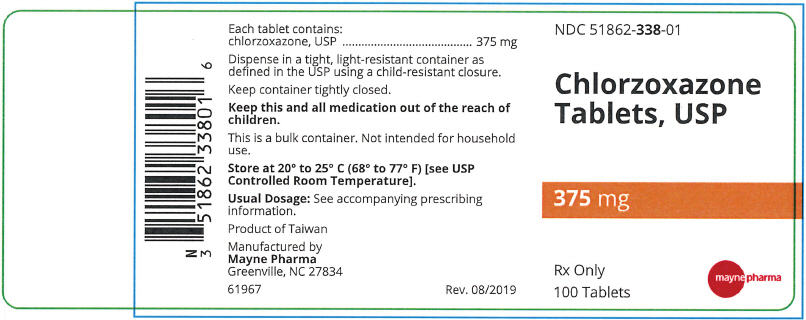
- NDC Code(s): 51862-338-01, 51862-339-01, 51862-340-01
- Packager: Mayne Pharma Commercial LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated July 6, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Each 375 mg Chlorzoxazone tablet contains: Chlorzoxazone USP 375 mg.
Each 500 mg Chlorzoxazone tablet contains: Chlorzoxazone USP 500 mg.
Each 750 mg Chlorzoxazone tablet contains: Chlorzoxazone USP 750 mg.
Chemical Name: 5-Chloro-2-benzoxazolinone.
Structural Formula:
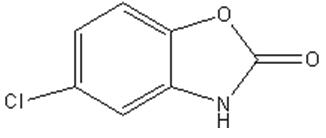
Molecular Formula: C 7H 4CINO 2
Molecular Weight: 169.56
Chlorzoxazone USP is a white or practically white, practically odorless, crystalline powder. Chlorzoxazone is slightly soluble in water; sparingly soluble in alcohol, in isopropyl alcohol, and in methanol; soluble in solutions of alkali hydroxides and ammonia.
Inactive ingredients:, croscarmellose sodium, docusate sodium, lactose monohydrate, magnesium stearate, microcrystalline cellulose, pregelatinized corn starch and sodium benzoate.
-
CLINICAL PHARMACOLOGY
Chlorzoxazone is a centrally-acting agent for painful musculoskeletal conditions. Data available from animal experiments as well as human study indicate that chlorzoxazone acts primarily at the level of the spinal cord and subcortical areas of the brain where it inhibits multisynaptic reflex arcs involved in producing and maintaining skeletal muscle spasm of varied etiology. The clinical result is a reduction of the skeletal muscle spasm with relief of pain and increased mobility of the involved muscles. Blood levels of chlorzoxazone can be detected in people during the first 30 minutes and peak levels may be reached, in the majority of the subjects, in about 1 to 2 hours after oral administration of chlorzoxazone. Chlorzoxazone is rapidly metabolized and is excreted in the urine, primarily in a conjugated form as the glucuronide. Less than one percent of a dose of chlorzoxazone is excreted unchanged in the urine in 24 hours.
-
INDICATIONS AND USAGE
Chlorzoxazone is indicated as an adjunct to rest, physical therapy, and other measures for the relief of discomfort associated with acute, painful musculoskeletal conditions. The mode of action of this drug has not been clearly identified, but may be related to its sedative properties. Chlorzoxazone does not directly relax tense skeletal muscles in man.
- CONTRAINDICATIONS
-
WARNINGS
Serious (including fatal) hepatocellular toxicity has been reported rarely in patients receiving chlorzoxazone. The mechanism is unknown but appears to be idiosyncratic and unpredictable. Factors predisposing patients to this rare event are not known. Patients should be instructed to report early signs and/ or symptoms of hepatoxicity such as fever, rash, anorexia, nausea, vomiting, fatigue, right upper quadrant pain, dark urine, or jaundice. Chlorzoxazone should be discontinued immediately and a physician consulted if any of these signs or symptoms develop. Chlorzoxazone use should also be discontinued if a patient develops abnormal liver enzymes (e.g., AST, ALT, alkaline phosphatase and bilirubin).
The concomitant use of alcohol or other central nervous system depressants may have an additive effect.
Usage in Pregnancy
The safe use of chlorzoxazone has not been established with respect to the possible adverse effects upon fetal development. Therefore, it should be used in women of childbearing potential only when, in the judgement of the physician, the potential benefits outweigh the possible risks.
-
PRECAUTIONS
Chlorzoxazone should be used with caution in patients with known allergies or with a history of allergic reactions to drugs. If sensitivity reaction occurs such as urticaria, redness, or itching of the skin, the drug should be stopped.
If any symptoms suggestive of liver dysfunction are observed, the drug should be discontinued.
-
ADVERSE REACTIONS
Chlorzoxazone containing products are usually well tolerated. It is possible in rare instances that chlorzoxazone may have been associated with gastrointestinal bleeding. Drowsiness, dizziness, light- headedness, malaise, or overstimulation may be noted by an occasional patient. Rarely, allergic-type skin rashes, petechiae, or ecchymoses may develop during treatment. Angioneurotic edema or anaphylactic reactions are extremely rare. There is no evidence that the drug will cause renal damage. Rarely, a patient may note discoloration of the urine resulting from a phenolic metabolite of chlorzoxazone. This finding is of no known clinical significance.
-
OVERDOSAGE
Symptoms
Initially, gastrointestinal disturbances such a nausea, vomiting, or diarrhea together with drowsiness, dizziness, lightheadedness or headache may occur. Early in the course there may be malaise or sluggishness followed by marked loss of muscle tone, making voluntary movement impossible. The deep tendon reflexes may be decreased or absent. The sensorium remains intact, and there is no peripheral loss of sensation. Respiratory depression may occur with rapid, irregular respiration and intercostals and substernal retraction. The blood pressure is lowered, but shock has not been observed.
Treatment
Gastric lavage or induction of emesis should be carried out, followed by administration of activated charcoal. Thereafter, treatment is entirely supportive. If respirations are depressed, oxygen and artificial respiration should be employed and a patent airway assured by use of an oropharyngeal airway or endotracheal tube. Hypotension may be counteracted by use of dextran, plasma, concentrated albumin or a vasopressor agent such as norepinephrine. Cholinergic drugs or analeptic drugs are of no value and should not be used.
-
DOSAGE AND ADMINISTRATION
Usual Adult Dosage
Chlorzoxazone tablets USP 375 mg
One tablet three or four times daily. If adequate response is not obtained with this dose, the 375 mg tablets may be increased to two tablets (750 mg) three or four times daily. As improvement occurs dosage can usually be reduced.
Chlorzoxazone tablets USP 500 mg
One tablet three or four times daily. If adequate response is not obtained with this dose, it may be increased to one and one-half tablets (750 mg) three or four times daily. As improvement occurs dosage can usually be reduced.
Chlorzoxazone tablets USP 750 mg
1/3 tablet (250 mg) three or four times daily. Initial dosage for painful musculoskeletal conditions should be 2/3 tablet (500 mg) three or four times daily. If adequate response is not obtained with this dose, it may be increased to one tablet (750 mg) three or four times daily. As improvement occurs dosage can usually be reduced.
-
HOW SUPPLIED
Chlorzoxazone tablets USP are supplied as follows:
375 mg
A white to off-white capsule shaped tablet, debossed "m338" on one side and plain on the other side, in bottles of 100 tablets, NDC 51862-338-01
500 mg
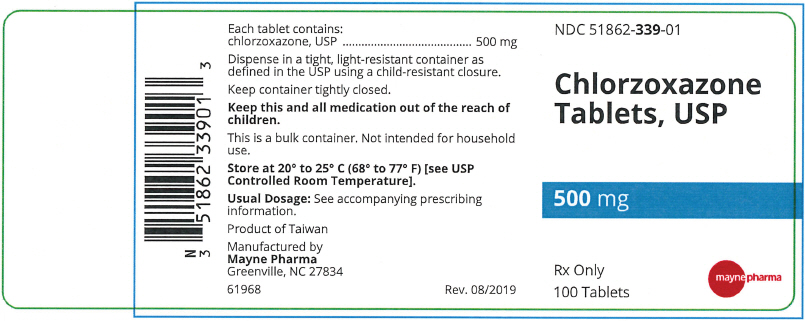
A white to off-white capsule shaped tablet with functional scoring on one side and debossed "m339" on one side and plain on the other side, in bottles of 100 tablets, NDC 51862-339-01
750 mg
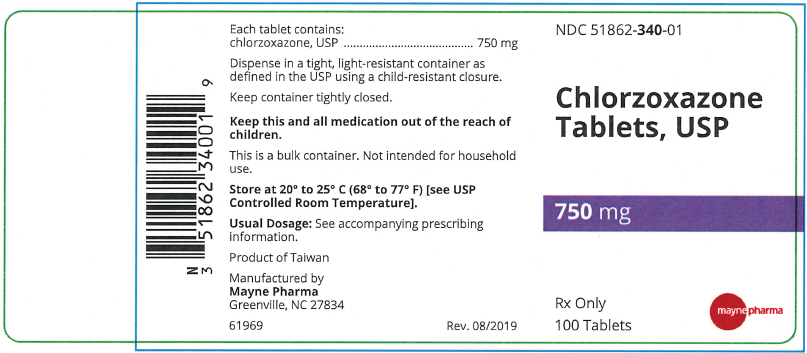
A white to off-white capsule shaped tablet with two functional scores on both sides of the tablet. The top side is debossed with "3", "4", and "0" on left, middle, and right portions, respectively. The bottom side is debossed with "m" on each tablet portion. The tablet has a functional bisect score on each band of the tablet. The tablets are provided in bottles of 100 tablets. NDC 51862-340-01
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 375 mg Tablet Bottle Label
- PRINCIPAL DISPLAY PANEL - 500 mg Tablet Bottle Label
- PRINCIPAL DISPLAY PANEL - 750 mg Tablet Bottle Label
-
INGREDIENTS AND APPEARANCE
CHLORZOXAZONE
chlorzoxazone tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:51862-338 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORZOXAZONE (UNII: H0DE420U8G) (CHLORZOXAZONE - UNII:H0DE420U8G) CHLORZOXAZONE 375 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) DOCUSATE SODIUM (UNII: F05Q2T2JA0) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) STARCH, CORN (UNII: O8232NY3SJ) SODIUM BENZOATE (UNII: OJ245FE5EU) Product Characteristics Color white Score no score Shape OVAL Size 17mm Flavor Imprint Code m338 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51862-338-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 07/31/2020 08/31/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA211849 07/31/2020 08/31/2022 CHLORZOXAZONE
chlorzoxazone tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:51862-339 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORZOXAZONE (UNII: H0DE420U8G) (CHLORZOXAZONE - UNII:H0DE420U8G) CHLORZOXAZONE 500 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) DOCUSATE SODIUM (UNII: F05Q2T2JA0) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) STARCH, CORN (UNII: O8232NY3SJ) SODIUM BENZOATE (UNII: OJ245FE5EU) Product Characteristics Color white Score 2 pieces Shape OVAL Size 17mm Flavor Imprint Code m339 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51862-339-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 07/31/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA211849 07/31/2020 CHLORZOXAZONE
chlorzoxazone tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:51862-340 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORZOXAZONE (UNII: H0DE420U8G) (CHLORZOXAZONE - UNII:H0DE420U8G) CHLORZOXAZONE 750 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) DOCUSATE SODIUM (UNII: F05Q2T2JA0) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) STARCH, CORN (UNII: O8232NY3SJ) SODIUM BENZOATE (UNII: OJ245FE5EU) Product Characteristics Color white Score 3 pieces Shape OVAL Size 20mm Flavor Imprint Code 340;mmm Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51862-340-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 07/31/2020 10/31/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA211849 07/31/2020 10/31/2022 Labeler - Mayne Pharma Commercial LLC (867220261) Establishment Name Address ID/FEI Business Operations Catalent Greenville, Inc. 118812386 manufacture(51862-338, 51862-339, 51862-340) , analysis(51862-338, 51862-339, 51862-340) , label(51862-338, 51862-339, 51862-340) , pack(51862-338, 51862-339, 51862-340)