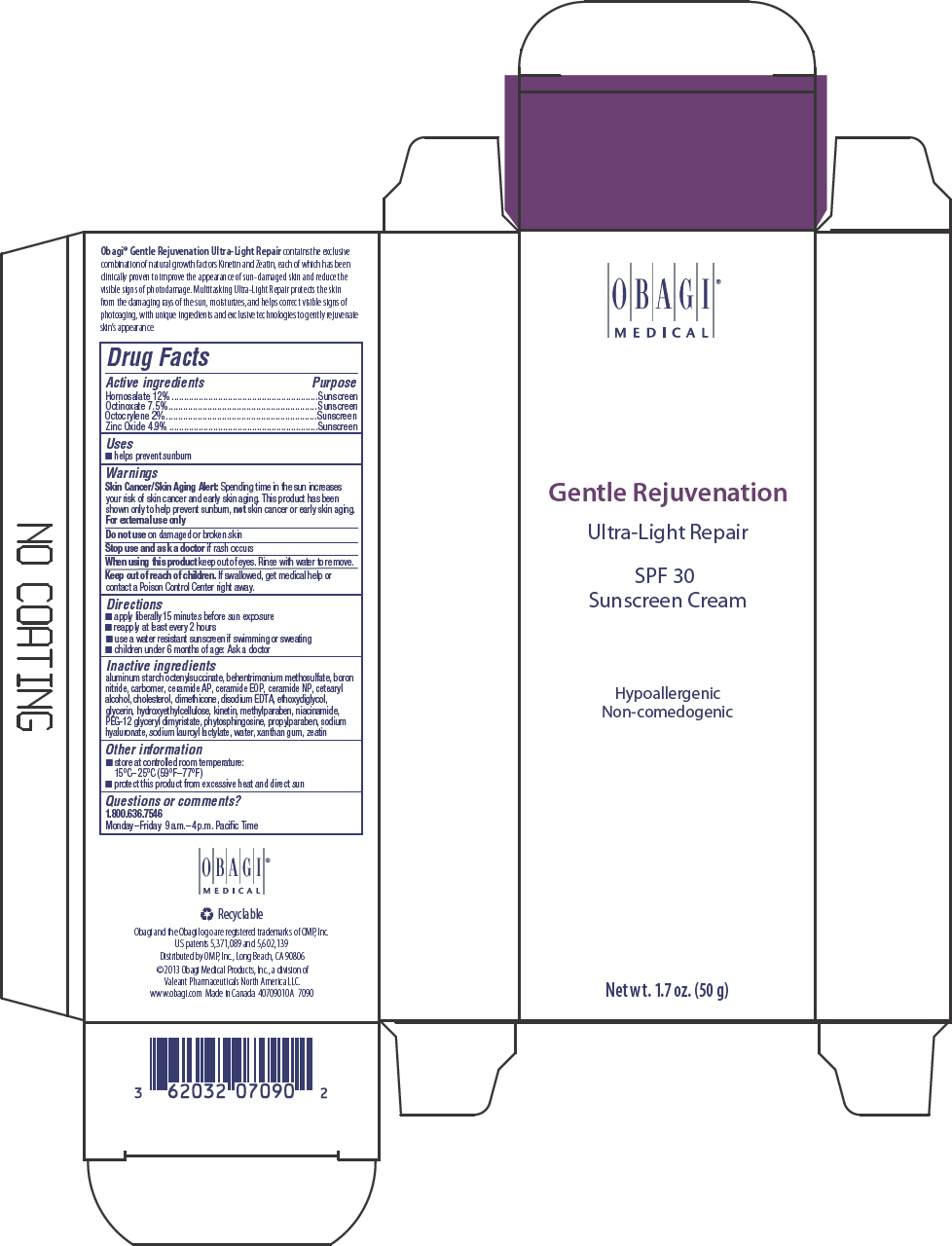
Label: GENTLE REJUVENATION ULTRA LIGHT REPAIR SPF 30 SUNSCREEN- homosalate, octinoxate, octocrylene, and zinc oxide cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 62032-131-70 - Packager: Obagi Medical Products, Inc., a division of Valeant Pharmaceuticals North America LLC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated October 15, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
- Warnings
- Directions
-
Inactive ingredients
aluminum starch octenylsuccinate, behentrimonium methosulfate, boron nitride, carbomer, ceramide AP, ceramide EOP, ceramide NP, cetearyl alcohol, cholesterol, dimethicone, disodium EDTA, ethoxydiglycol, glycerin, hydroxyethylcellulose, kinetin, methylparaben, niacinamide, PEG-12 glyceryl dimyristate, phytosphingosine, propylparaben, sodium hyaluronate, sodium lauroyl lactylate, water, xanthan gum, zeatin
- Other information
- Questions or comments?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 50 g Tube Carton
-
INGREDIENTS AND APPEARANCE
GENTLE REJUVENATION ULTRA LIGHT REPAIR SPF 30 SUNSCREEN
homosalate, octinoxate, octocrylene, and zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62032-131 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 120 mg in 1 g OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 75 mg in 1 g OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 20 mg in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 49 mg in 1 g Inactive Ingredients Ingredient Name Strength ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) BEHENTRIMONIUM METHOSULFATE (UNII: 5SHP745C61) BORON NITRIDE (UNII: 2U4T60A6YD) CARBOMER HOMOPOLYMER TYPE B (ALLYL SUCROSE CROSSLINKED) (UNII: Z135WT9208) CERAMIDE 6 II (UNII: F1X8L2B00J) CERAMIDE 1 (UNII: 5THT33P7X7) CERAMIDE 3 (UNII: 4370DF050B) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) CHOLESTEROL (UNII: 97C5T2UQ7J) DIMETHICONE 100 (UNII: RO266O364U) EDETATE DISODIUM (UNII: 7FLD91C86K) DIETHYLENE GLYCOL MONOETHYL ETHER (UNII: A1A1I8X02B) GLYCERIN (UNII: PDC6A3C0OX) KINETIN (UNII: P39Y9652YJ) METHYLPARABEN (UNII: A2I8C7HI9T) NIACINAMIDE (UNII: 25X51I8RD4) PEG-12 GLYCERYL DIMYRISTATE (UNII: VS4W16AQ3X) PHYTOSPHINGOSINE (UNII: GIN46U9Q2Q) PROPYLPARABEN (UNII: Z8IX2SC1OH) HYALURONATE SODIUM (UNII: YSE9PPT4TH) SODIUM LAUROYL LACTYLATE (UNII: 7243K85WFO) WATER (UNII: 059QF0KO0R) XANTHAN GUM (UNII: TTV12P4NEE) ZEATIN (UNII: 7I6OOJ9GR6) Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62032-131-70 1 in 1 CARTON 1 50 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 10/15/2013 Labeler - Obagi Medical Products, Inc., a division of Valeant Pharmaceuticals North America LLC. (790553353) Establishment Name Address ID/FEI Business Operations LABORATOIRE DR RENAUD INC. 202501565 MANUFACTURE(62032-131) , LABEL(62032-131) , PACK(62032-131)