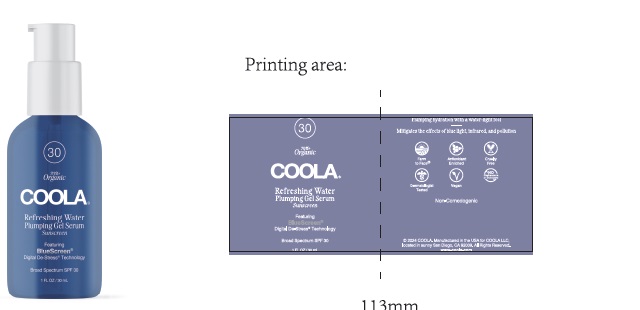
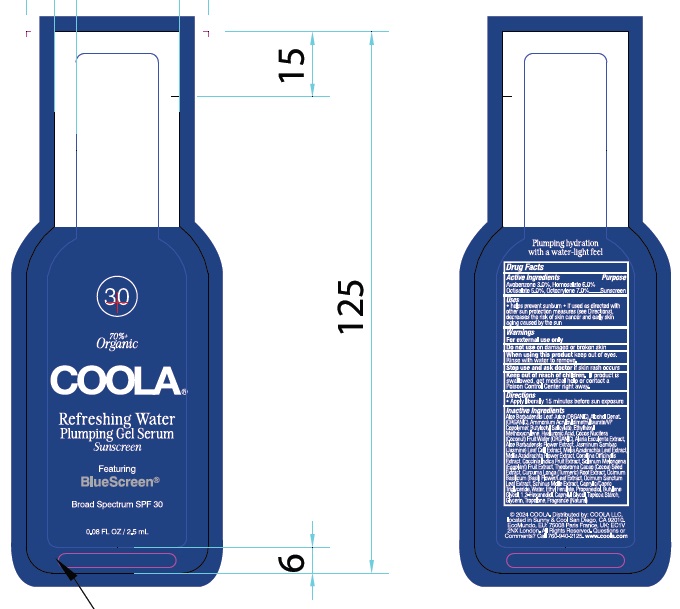
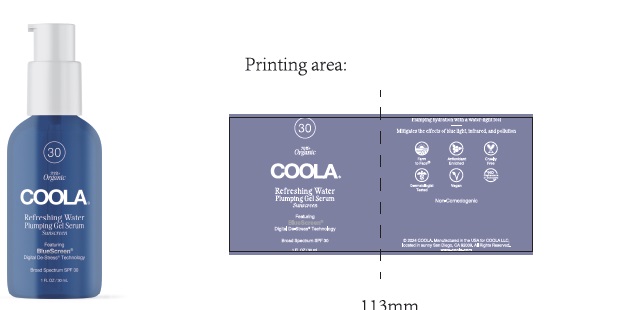
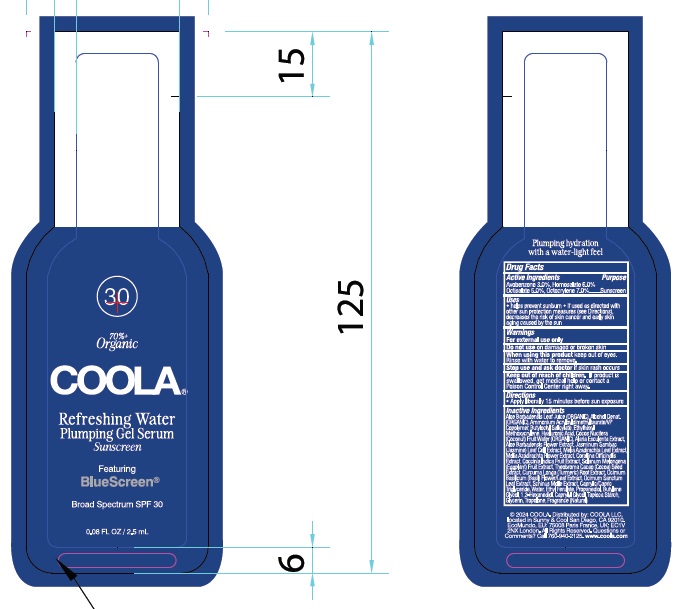
Label: COOLA REFRESHING WATER PLUMPING GEL SERUM SPF 30- avobenzone, homosalate, octisalate, octocrylene gel
- NDC Code(s): 79753-081-01, 79753-081-02, 79753-081-03
- Packager: COOLA LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 29, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- INDICATIONS & USAGE
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions
● apply liberally 15 minutes before sun exposure.
● reapply at least every 2 hours
● use a water resistant sunscreen if wimming or sweating
Sun Protection Measures. Spending time in the sun
increases your risk of skin cancer and early skin aging. To
decrease this risk, regularly use a sunscreen with a broad
spection SPF of 15 or higher and other sun protection
measures including:
● limit time in the sun, especially from: 10 a.m. - 2 p.m.
● wear long-sleeve shirts, pants, hats, and sunglasses
●children under 6 months: Ask a doctor -
INACTIVE INGREDIENT
Inactive Ingredients
Aloe Barbadensis Leaf Juice (ORGANIC), Alcohol Denat (ORGANIC),
Ammonium Acryloyldimethyltaurate/VP Copolymer Butyloctyl
Salicylate, Ethylhexyl Methoxycrylene, Hyaluronic Acid, Cocos Nucifera
(Coconut) Fruit Water (ORGANIC), Alaria Esculenta Extract, Aloe
Barbadensis Flower Extract, Jasminum Sambac (Jasmine) Leaf Cell
Extract, Melia Azadirachta Leaf Extract, Melia Azadirachta Flower Extract,
Corallina Officinalis Extract, Coccinia Indica Fruit Extract, Solanum
Melongena (Eggplant) Fruit Extract, Theobroma Cacao (Cocoa) Seed
Extract, Curcuma Longa (Turmeric) Root Extract, Ocimum Basilicum
(Basil) Flower/Leaf Extract, Ocimum Sanctum Leaf Extract, Schinus
Molle Extract, Caprylic/Capric Triglyceride, Water, Ethyl Ferulate,
Propanediol, Butylene Glycol, 1,2-Hexanediol, Caprylyl Glycol, Triethyl
Citrate*, Tapioca Starch, Glycerin, Gamma-Nonalactone*, Tropolone,
Juniperus Mexicana Oil*, Gamma-Octalactone*, Benzyl Acetate*,
Phenylethyl Alcohol*, Mimosa Tenuiflora Bark Extract*, Vanillin*,
Heliotropine*, Anisaldehyde*, Delta-Decalactone*, Methylbenzyl
Acetate*, Ethyl Butyrate*, Methyl Anthranilate*, Methyl Benzoate**Plant derived scent constituent. See coola.com for additional detail.
- OTHER SAFETY INFORMATION
- QUESTIONS
- COOLA Refreshing Water Plumping Gel Serum - Labeling
-
INGREDIENTS AND APPEARANCE
COOLA REFRESHING WATER PLUMPING GEL SERUM SPF 30
avobenzone, homosalate, octisalate, octocrylene gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:79753-081 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3.0 g in 100 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 6.0 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5.0 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 7.0 g in 100 mL Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) ALCOHOL (UNII: 3K9958V90M) AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER (UNII: W59H9296ZG) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) ETHYLHEXYL METHOXYCRYLENE (UNII: S3KFG6Q5X8) HYALURONIC ACID (UNII: S270N0TRQY) COCONUT WATER (UNII: 267F5Y81NT) ALARIA ESCULENTA (UNII: EJ9JK8J58D) ALOE VERA FLOWER (UNII: 575DY8C1ER) JASMINUM OFFICINALE LEAF (UNII: 6OG06384ID) AZADIRACHTA INDICA LEAF (UNII: HKY915780T) AZADIRACHTA INDICA FLOWER (UNII: 3TE8A92UPM) CORALLINA OFFICINALIS (UNII: 4004498D06) COCCINIA GRANDIS FRUIT (UNII: VLJ6WOT3K5) EGGPLANT (UNII: W5K7RAS4VK) COCOA (UNII: D9108TZ9KG) TURMERIC (UNII: 856YO1Z64F) HOLY BASIL LEAF (UNII: SCJ765569P) OCIMUM BASILICUM FLOWERING TOP (UNII: 7SAB275FP2) SCHINUS MOLLE FRUITING TOP (UNII: T8L6O1KSB4) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) WATER (UNII: 059QF0KO0R) ETHYL FERULATE (UNII: 5B8915UELW) PROPANEDIOL (UNII: 5965N8W85T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) CAPRYLYL GLYCOL (UNII: 00YIU5438U) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) STARCH, TAPIOCA (UNII: 24SC3U704I) GLYCERIN (UNII: PDC6A3C0OX) .GAMMA.-NONALACTONE (UNII: I1XGH66S8P) TROPOLONE (UNII: 7L6DL16P1T) JUNIPERUS DEPPEANA WOOD OIL (UNII: 4739QA5686) .GAMMA.-OCTALACTONE (UNII: UHD6M52X0K) BENZYL ACETATE (UNII: 0ECG3V79ZJ) PHENYLETHYL ALCOHOL (UNII: ML9LGA7468) MIMOSA TENUIFLORA BARK (UNII: 515MQE449I) VANILLIN (UNII: CHI530446X) PIPERONAL (UNII: KE109YAK00) P-ANISALDEHYDE (UNII: 9PA5V6656V) .DELTA.-DECALACTONE (UNII: CNA0S5T234) METHYLBENZYL ACETATE (UNII: P4RVP859F5) ETHYL BUTYRATE (UNII: UFD2LZ005D) METHYL ANTHRANILATE (UNII: 981I0C1E5W) METHYL BENZOATE (UNII: 6618K1VJ9T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:79753-081-01 1 in 1 CARTON 01/01/2024 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:79753-081-02 7 mL in 1 TUBE; Type 0: Not a Combination Product 01/01/2024 3 NDC:79753-081-03 2.5 mL in 1 POUCH; Type 0: Not a Combination Product 01/01/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 01/01/2024 Labeler - COOLA LLC (956990290) Registrant - COOLA LLC (956990290)