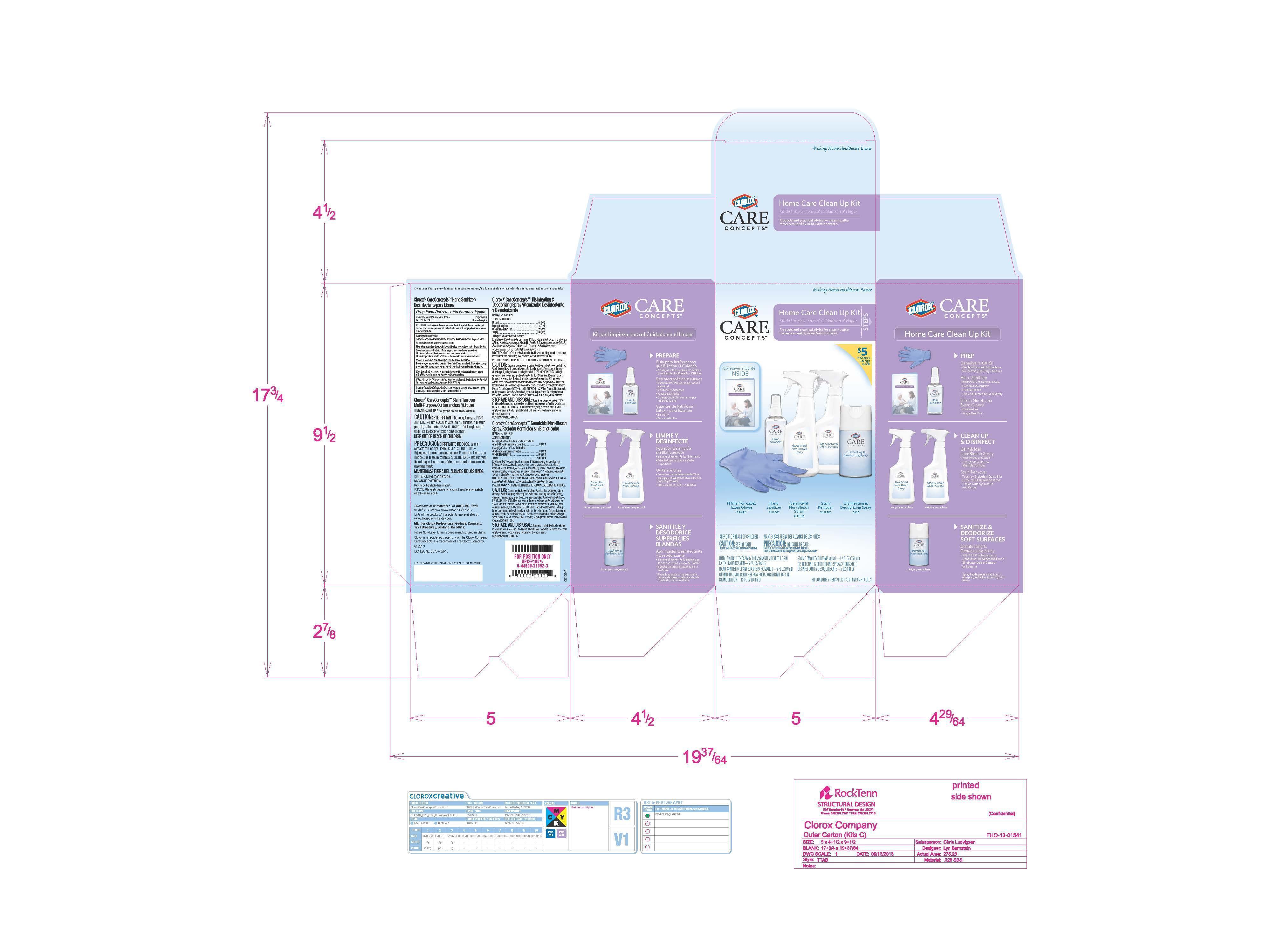
Label: CLOROX CARE CONCEPTS- kit
- NHRIC Code(s): 26509-0005-1
-
Contains inactivated NDC Code(s)
NDC Code(s): 26509-0002-1 - Packager: The Clorox Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Exempt device
Drug Label Information
Updated August 21, 2014
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Use
- Warnings
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other Information
- Inactive Ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CLOROX CARE CONCEPTS
patient personal hygiene kit kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NHRIC:26509-0005 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:26509-0005-1 1 in 1 KIT 04/07/2014 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE, SPRAY 59 mL Part 2 0 PACKAGE 1 Part 1 of 2 CLOROX CARE CONCEPTS HAND SANITIZER
alcohol solutionProduct Information Item Code (Source) NDC:26509-0002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 67 mL in 100 mL Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) ISOPROPYL ALCOHOL (UNII: ND2M416302) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL LAURATE (UNII: Y98611C087) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:26509-0002-1 59 mL in 1 BOTTLE, SPRAY Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 04/07/2014 Part 2 of 2 NITRILE NON-LATEX GLOVES
patient examination gloveProduct Information Item Code (Source) GS1:8438995971 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 5 in 1 PACKAGE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date premarket notification K051378 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date exempt device ABC 04/07/2014 Labeler - The Clorox Company (009138033) Registrant - The Clorox Company (009138033)