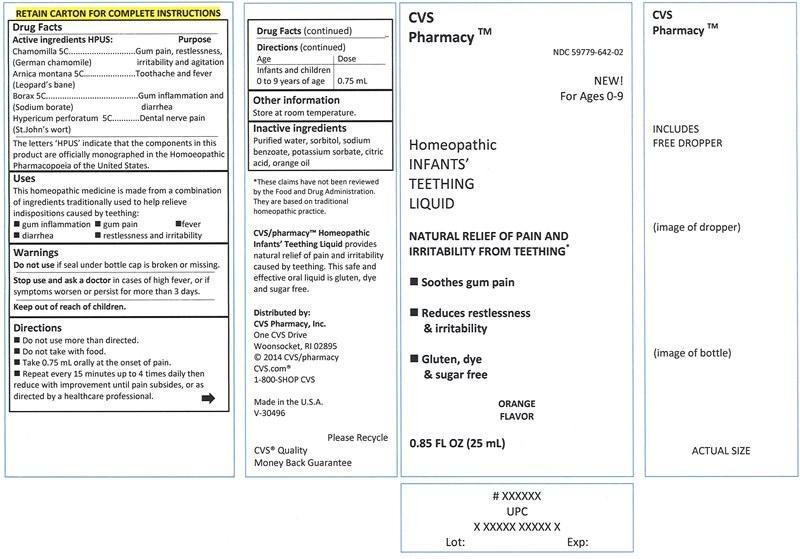
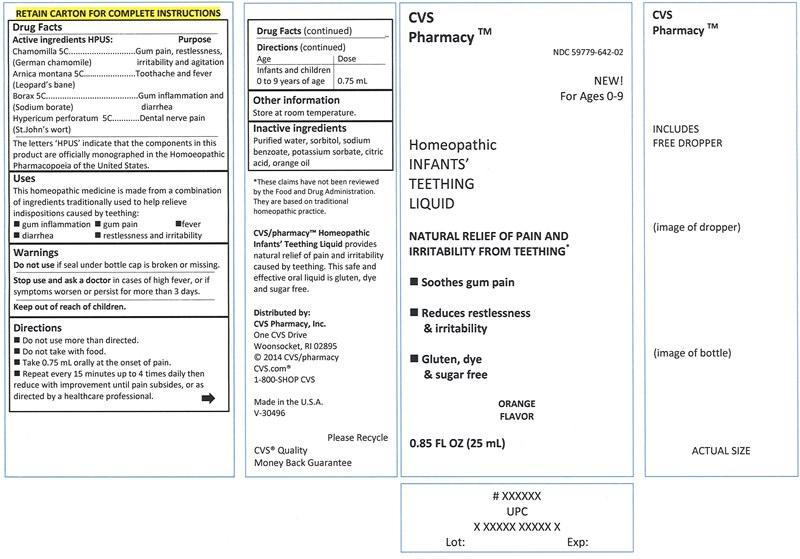
Label: INFANTS TEETHING LIQUID- chamomilla, arnica montana, borax, hypericum perforatum liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 59779-642-02 - Packager: CVS PHARMACY
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated February 25, 2014
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients HPUS:
-
Purpose
Chamomilla (German chamomile) 5C .....................................................gum pain, restlessness, irritability and agitation
Arnica montana (Leopard's bane) 5C ......................................................toothache and fever
Borax (Sodium borate) 5C .....................................................................gum inflammation and diarrhea
Hypericum perforatum (St.John's wort) 5C ..............................................dental nerve pain
- REFERENCES
-
Uses
This homeopathic medicine is made from a combination of ingredients traditionally used to help relieve indispositions caused by teething:
- gum inflammation
- gum pain
- fever
- diarrhea
- restlessness and irritability
*Thses claims have not been reviewed by the Food and Drug Administration. They are based on traditional homeopathic practice.
- Warnings
- Directions
- Other information
- Inactive ingredients
- REFERENCES
- CARTON
-
INGREDIENTS AND APPEARANCE
INFANTS TEETHING LIQUID
chamomilla, arnica montana, borax, hypericum perforatum liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59779-642 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHAMOMILE (UNII: FGL3685T2X) (CHAMOMILE - UNII:FGL3685T2X) CHAMOMILE 5 [hp_C] in 25 mL ARNICA MONTANA (UNII: O80TY208ZW) (ARNICA MONTANA - UNII:O80TY208ZW) ARNICA MONTANA 5 [hp_C] in 25 mL SODIUM BORATE (UNII: 91MBZ8H3QO) (BORATE ION - UNII:44OAE30D22) SODIUM BORATE 5 [hp_C] in 25 mL HYPERICUM PERFORATUM (UNII: XK4IUX8MNB) (HYPERICUM PERFORATUM - UNII:XK4IUX8MNB) HYPERICUM PERFORATUM 5 [hp_C] in 25 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SORBITOL (UNII: 506T60A25R) SODIUM BENZOATE (UNII: OJ245FE5EU) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) Product Characteristics Color Score Shape Size Flavor ORANGE Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59779-642-02 1 in 1 CARTON 1 25 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 02/25/2014 Labeler - CVS PHARMACY (062312574) Registrant - HOMEOLAB USA INC (202032533) Establishment Name Address ID/FEI Business Operations HOMEOLAB USA INC 202032533 manufacture(59779-642)