Label: MEMANTINE HYDROCHLORIDE tablet, film coated
-
NDC Code(s):
70771-1119-0,
70771-1119-1,
70771-1119-2,
70771-1119-4, view more70771-1119-5, 70771-1119-6, 70771-1120-0, 70771-1120-1, 70771-1120-2, 70771-1120-4, 70771-1120-5, 70771-1120-6
- Packager: Zydus Lifesciences Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 5, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
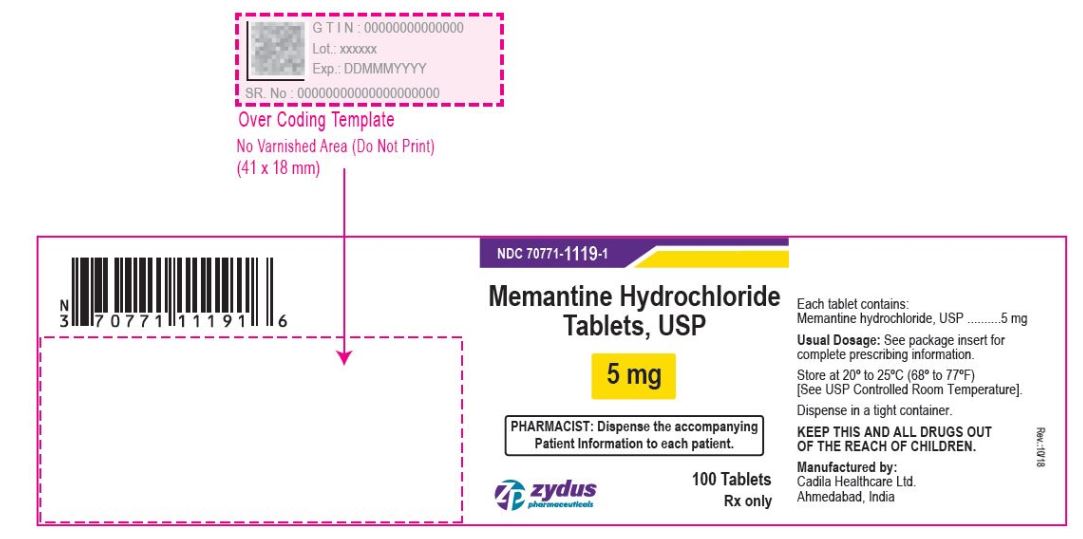
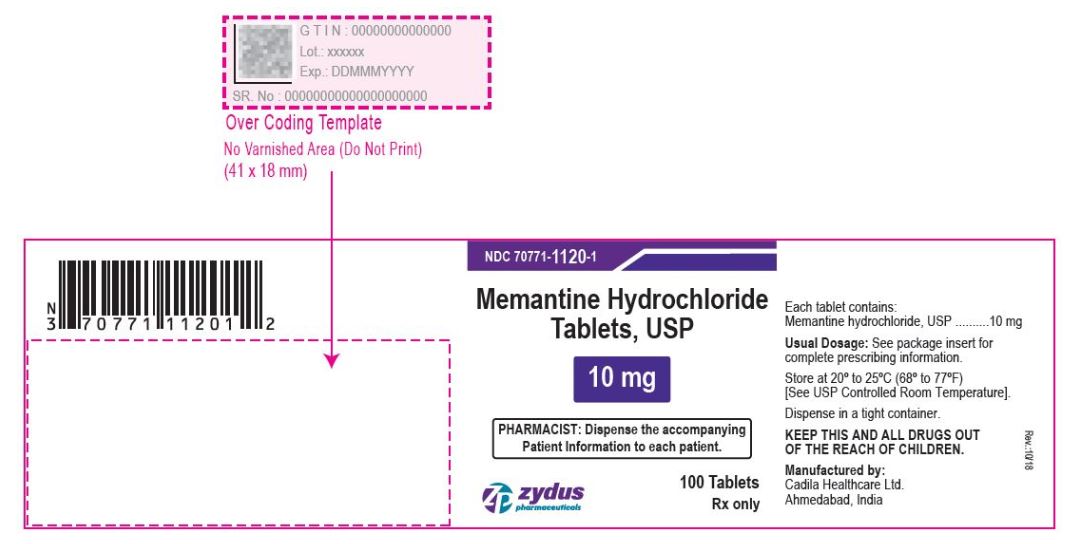
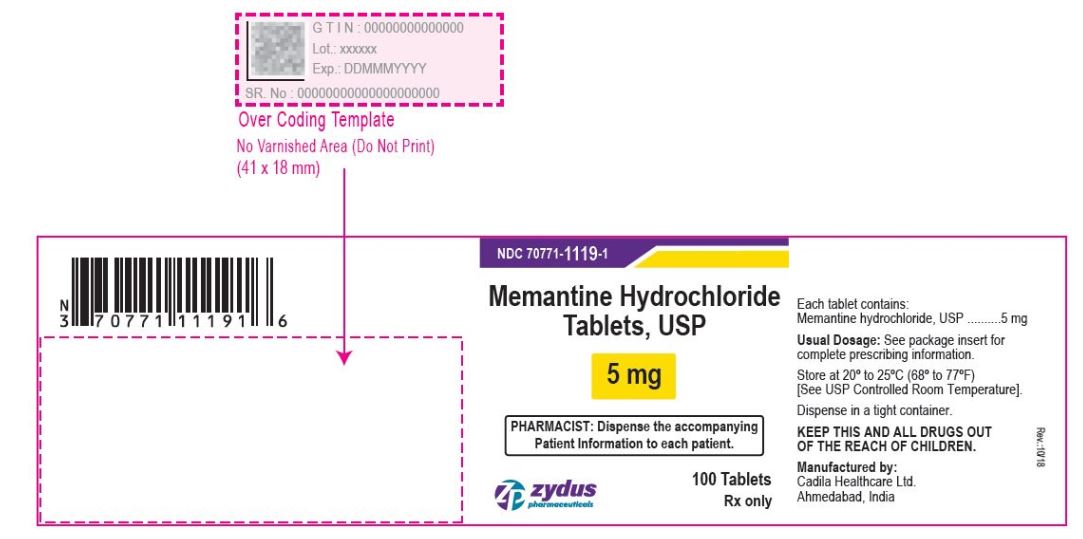
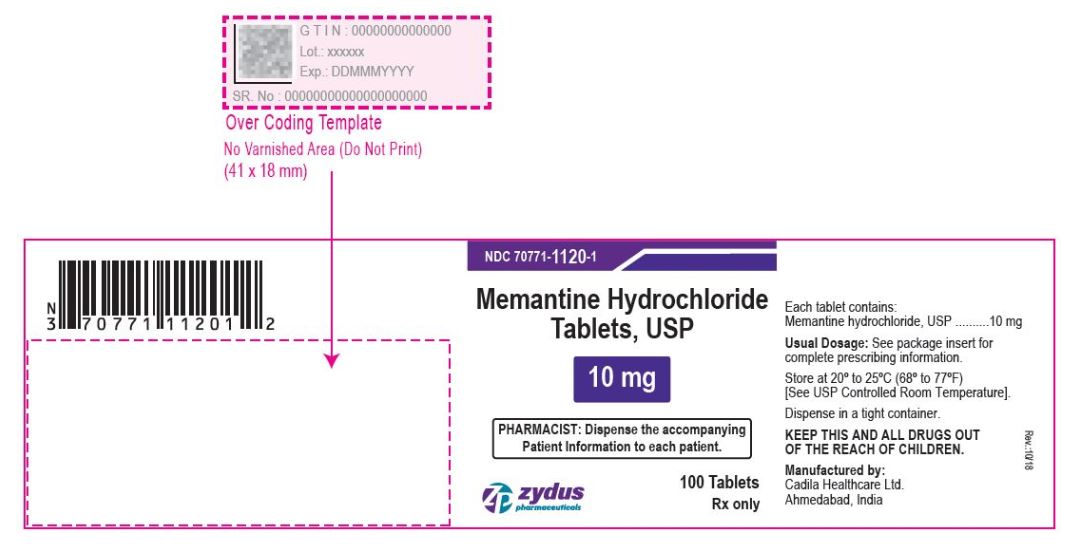
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MEMANTINE HYDROCHLORIDE
memantine hydrochloride tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70771-1119 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MEMANTINE HYDROCHLORIDE (UNII: JY0WD0UA60) (MEMANTINE - UNII:W8O17SJF3T) MEMANTINE HYDROCHLORIDE 5 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) HYPROMELLOSES (UNII: 3NXW29V3WO) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) MAGNESIUM STEARATE (UNII: 70097M6I30) POVIDONE (UNII: FZ989GH94E) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) Product Characteristics Color WHITE (WHITE TO OFF-WHITE) Score no score Shape CAPSULE (CAPSULE) Size 9mm Flavor Imprint Code ZF;41 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70771-1119-4 100 in 1 CARTON 09/28/2017 1 NDC:70771-1119-2 1 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:70771-1119-6 60 in 1 BOTTLE; Type 0: Not a Combination Product 09/28/2017 3 NDC:70771-1119-1 100 in 1 BOTTLE; Type 0: Not a Combination Product 09/28/2017 4 NDC:70771-1119-5 500 in 1 BOTTLE; Type 0: Not a Combination Product 09/28/2017 5 NDC:70771-1119-0 1000 in 1 BOTTLE; Type 0: Not a Combination Product 09/28/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090961 09/28/2017 MEMANTINE HYDROCHLORIDE
memantine hydrochloride tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70771-1120 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MEMANTINE HYDROCHLORIDE (UNII: JY0WD0UA60) (MEMANTINE - UNII:W8O17SJF3T) MEMANTINE HYDROCHLORIDE 10 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) HYPROMELLOSES (UNII: 3NXW29V3WO) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) MAGNESIUM STEARATE (UNII: 70097M6I30) POVIDONE (UNII: FZ989GH94E) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) Product Characteristics Color WHITE (WHITE TO OFF-WHITE) Score no score Shape CAPSULE (CAPSULE) Size 11mm Flavor Imprint Code ZF;40 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70771-1120-6 60 in 1 BOTTLE; Type 0: Not a Combination Product 09/28/2017 2 NDC:70771-1120-1 100 in 1 BOTTLE; Type 0: Not a Combination Product 09/28/2017 3 NDC:70771-1120-5 500 in 1 BOTTLE; Type 0: Not a Combination Product 09/28/2017 4 NDC:70771-1120-0 1000 in 1 BOTTLE; Type 0: Not a Combination Product 09/28/2017 5 NDC:70771-1120-4 1000 in 1 CARTON 09/28/2017 5 NDC:70771-1120-2 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090961 09/28/2017 Labeler - Zydus Lifesciences Limited (918596198) Registrant - Zydus Lifesciences Limited (918596198) Establishment Name Address ID/FEI Business Operations Zydus Lifesciences Limited 918596198 ANALYSIS(70771-1119, 70771-1120) , MANUFACTURE(70771-1119, 70771-1120)