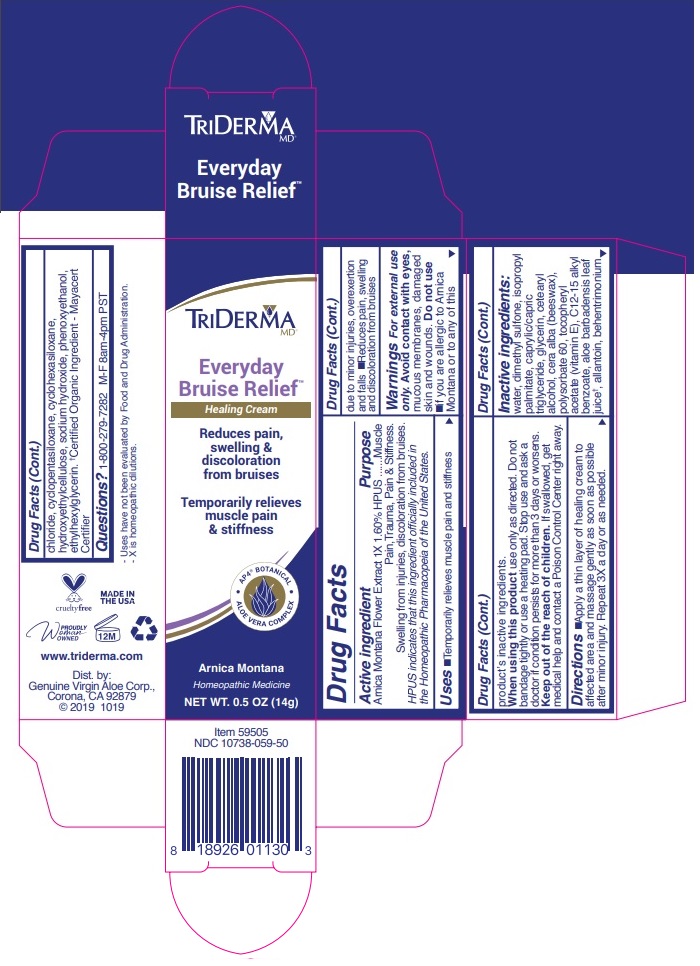
Label: TRIDERMA EVERYDAY BRUISE RELIEF- arnica montana cream
-
NDC Code(s):
10738-059-20,
10738-059-25,
10738-059-41,
10738-059-50, view more10738-059-55
- Packager: Genuine Virgin Aloe Corporation
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated February 2, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Purpose
- INDICATIONS & USAGE
-
WARNINGS
Warnings For external use only.
Avoid contact with eyes, mucous membranes, damaged skin and wounds.
Do not use •if you are allergic to Arnica Montana or to any of this product’s inactive ingredients.
When using this product use only as directed. Do not bandage tightly or use a heating pad. Stop use and ask a doctor if condition persists for more than 3 days or worsens.
- DOSAGE & ADMINISTRATION
-
INACTIVE INGREDIENT
Inactive ingredients: water, dimethyl sulfone, isopropyl palmitate, caprylic/capric triglyceride, glycerin, cetearyl alcohol, cera alba (beeswax), polysorbate 60, tocopheryl acetate (vitamin E), C12-15 alkyl benzoate, aloe barbadensis leaf juice †, allantoin, behentrimonium chloride, cyclopentasiloxane, cyclohexasiloxane, hydroxyethylcellulose, sodium hydroxide, phenoxyethanol, ethylhexylglycerin. †Certified Organic Ingredient - Mayacert Certifier
- QUESTIONS
-
SPL UNCLASSIFIED SECTION
Reduces pain, swelling & discoloration from bruises Temporarily relieves muscle pain & stiffness
AP4 BOTANICAL
ALOE VERA COMPLEX
Arnica Montana
Homeopathic Medicine
Maximum Strength
Healing Botanicals
This is the perfect ‘must-have’ all-purpose healing cream for every medicine cabinet.
It provides soothing relief for dry, cracked skin, minor cuts, scrapes, burns, hard-to-heal™ skin irritations and much more.
Infused with our AP4 ® Organic Aloe* Complex, vitamins, minerals and essential botanicals, it delivers fast results without harmful side effects
www.triderma.com
MADE IN THE USA
Dist. by: Genuine Virgin Aloe Corp.,
Corona, CA 92879 © 2019 0919
Dermatologist Tested
Non-Greasy • No Cortisone Or Parabens
No Added Fragrance
- Uses have not been evaluated by Food and Drug Administration.
- X is homeopathic dilutions.
EXPERTS IN SKIN HEALING SINCE 1992
TriDerma MD ® provides healing solutions for irritated skin without harmful side effects.
It all began after one woman struggled to find safe and effective healing for her own skin problem.
This journey led to the creation of our proprietary AP4® Organic Aloe* Complex which is key to all our products.
QUALITY YOU CAN FEEL, RESULTS YOU CAN SEE.®
Each product is made using this powerful complex plus nature’s best botanicals and clinically proven ingredients.
We are passionate in our pursuit to create fast healing products that work.Everyday Bruise Relief™ soothing cream combines some of nature’s best botanicals to help improve the look of bruised skin. Daily use provides hydration for all skin types & restores the skin to look more healthy.
Arnica Montana - known to help soothe irritated skin
Allantoin & Vitamin E
- Packaging
- Packaging
- Packaging
- Packaging
- Packaging
-
INGREDIENTS AND APPEARANCE
TRIDERMA EVERYDAY BRUISE RELIEF
arnica montana creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10738-059 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARNICA MONTANA (UNII: O80TY208ZW) (ARNICA MONTANA - UNII:O80TY208ZW) ARNICA MONTANA 1.6 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIMETHYL SULFONE (UNII: 9H4PO4Z4FT) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) GLYCERIN (UNII: PDC6A3C0OX) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) WHITE WAX (UNII: 7G1J5DA97F) POLYSORBATE 60 (UNII: CAL22UVI4M) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) ALOE VERA LEAF (UNII: ZY81Z83H0X) ALLANTOIN (UNII: 344S277G0Z) BEHENTRIMONIUM CHLORIDE (UNII: X7GNG3S47T) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) CYCLOMETHICONE 6 (UNII: XHK3U310BA) HYDROXYETHYL CELLULOSE (100 MPA.S AT 2%) (UNII: R33S7TK2EP) SODIUM HYDROXIDE (UNII: 55X04QC32I) PHENOXYETHANOL (UNII: HIE492ZZ3T) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10738-059-20 1 in 1 CARTON 08/30/2019 1 NDC:10738-059-25 62 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:10738-059-50 1 in 1 CARTON 08/30/2019 2 NDC:10738-059-55 14 g in 1 TUBE; Type 0: Not a Combination Product 3 NDC:10738-059-41 113 g in 1 JAR; Type 0: Not a Combination Product 08/30/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/30/2019 Labeler - Genuine Virgin Aloe Corporation (961374147) Establishment Name Address ID/FEI Business Operations Genuine Virgin Aloe Corporation 961374147 manufacture(10738-059)