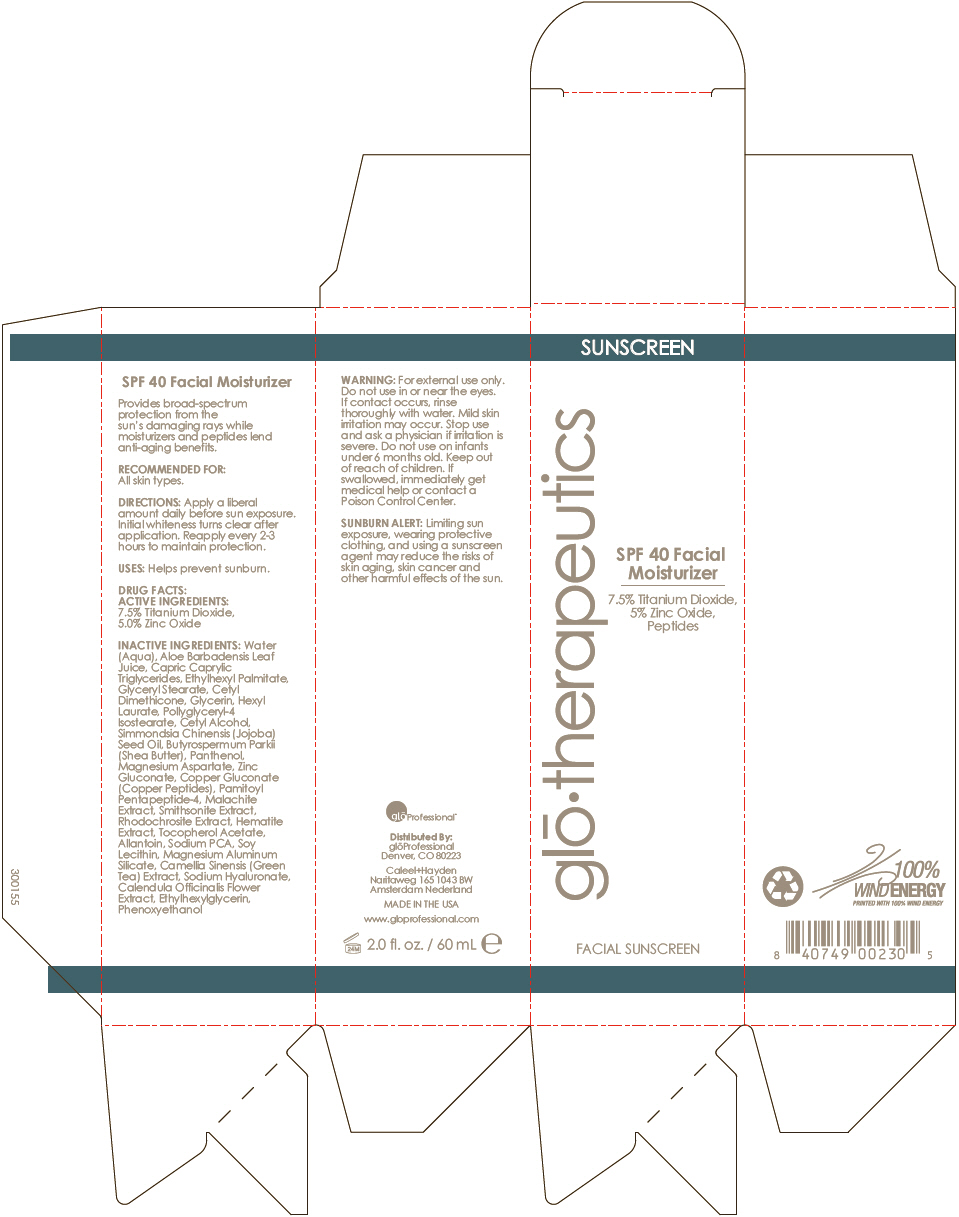
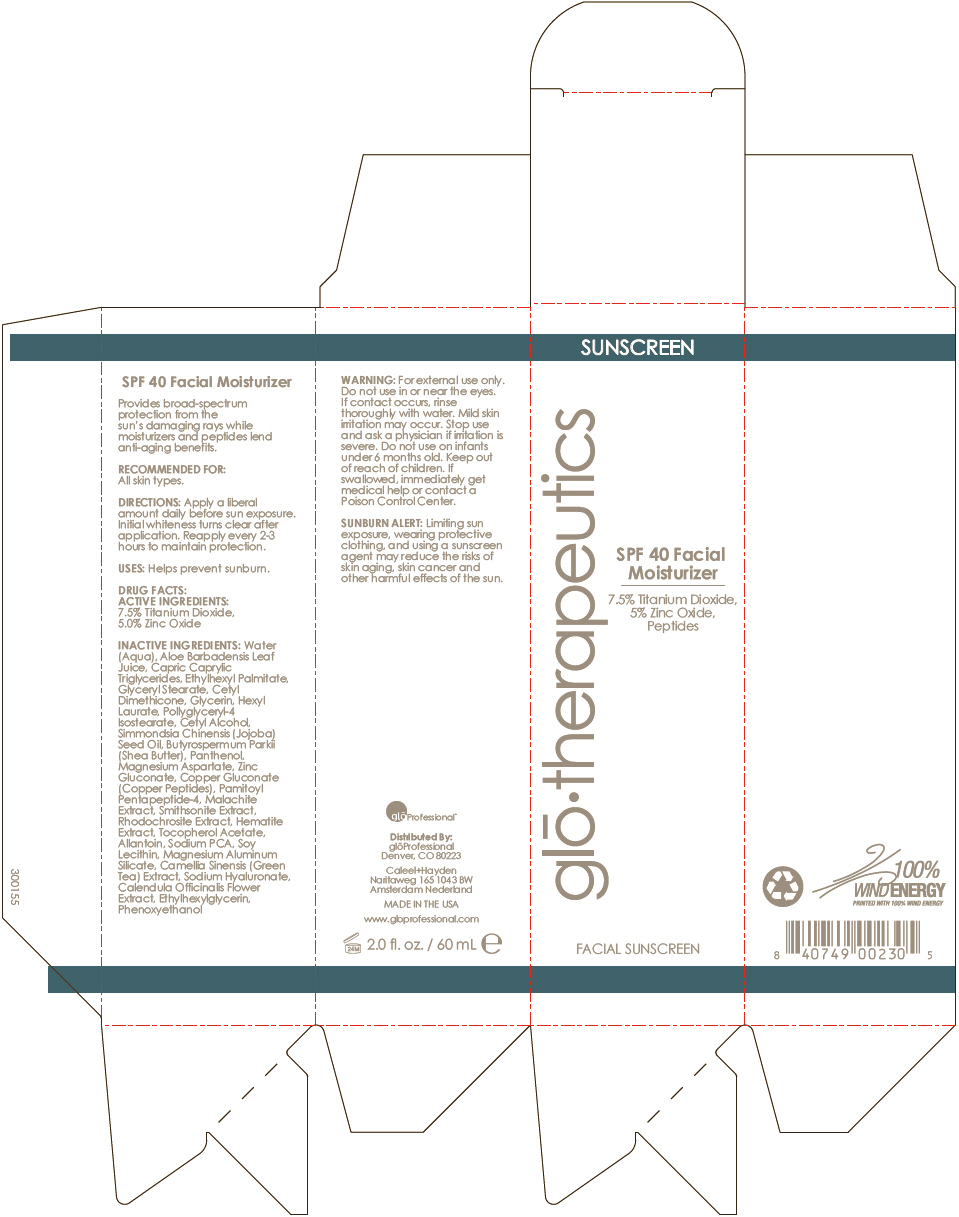
Label: SPF-40 FACIAL MOISTURIZER- zinc oxide and titanium dioxide lotion
-
Contains inactivated NDC Code(s)
NDC Code(s): 55789-0101-1 - Packager: Mineral Fusion brands
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated April 5, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- PURPOSE
- RECOMMENDED FOR
- DIRECTIONS
- USES
- ACTIVE INGREDIENTS
-
INACTIVE INGREDIENTS
Water (Aqua), Aloe Barbadensis Leaf Juice, Capric Caprylic Triglycerides, Ethylhexyl Palmitate, Glyceryl Stearate, Cetyl Dimethicone, Glycerin, Hexyl Laurate, Pollyglyceryl-4 Isostearate, Cetyl Alcohol, Simmondsia Chinensis (Jojoba) Seed Oil, Butyrospermum Parkii (Shea Butter), Panthenol, Magnesium Aspartate, Zinc Gluconate, Copper Gluconate (Copper Peptides), Pamitoyl Pentapeptide-4, Malachite Extract, Smithsonite Extract, Rhodochrosite Extract, Hematite Extract, Tocopherol Acetate, Allantoin, Sodium PCA, Soy Lecithin, Magnesium Aluminum Silicate, Camellia Sinensis (Green Tea) Extract, Sodium Hyaluronate, Calendula Officinalis Flower Extract, Ethylhexylglycerin, Phenoxyethanol
- WARNING
- SUNBURN ALERT
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 60 mL Tube Carton
-
INGREDIENTS AND APPEARANCE
SPF-40 FACIAL MOISTURIZER
zinc oxide and titanium dioxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:55789-0101 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 5 g in 100 mL Titanium dioxide (UNII: 15FIX9V2JP) (Titanium dioxide - UNII:15FIX9V2JP) Titanium dioxide 7.5 g in 100 mL Inactive Ingredients Ingredient Name Strength ethylhexyl palmitate (UNII: 2865993309) glyceryl monostearate (UNII: 230OU9XXE4) cetyl dimethicone 25 (UNII: U4AS1BW4ZB) glycerin (UNII: PDC6A3C0OX) hexyl laurate (UNII: 4CG9F9W01Q) aloe vera leaf (UNII: ZY81Z83H0X) polyglyceryl-4 isostearate (UNII: 820DPX33S7) medium-chain triglycerides (UNII: C9H2L21V7U) cetyl alcohol (UNII: 936JST6JCN) shea butter (UNII: K49155WL9Y) panthenol (UNII: WV9CM0O67Z) magnesium aspartate (UNII: R17X820ROL) zinc gluconate (UNII: U6WSN5SQ1Z) jojoba oil (UNII: 724GKU717M) tocopherol (UNII: R0ZB2556P8) copper gluconate (UNII: RV823G6G67) hyaluronate sodium (UNII: YSE9PPT4TH) palmitoyl pentapeptide-4 (UNII: KK181SM5JG) ethylhexylglycerin (UNII: 147D247K3P) allantoin (UNII: 344S277G0Z) sodium pyrrolidone carboxylate (UNII: 469OTG57A2) lecithin, soybean (UNII: 1DI56QDM62) magnesium aluminum silicate (UNII: 6M3P64V0NC) green tea leaf (UNII: W2ZU1RY8B0) phenoxyethanol (UNII: HIE492ZZ3T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55789-0101-1 60 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 12/10/2012 Labeler - Mineral Fusion brands (831770032)