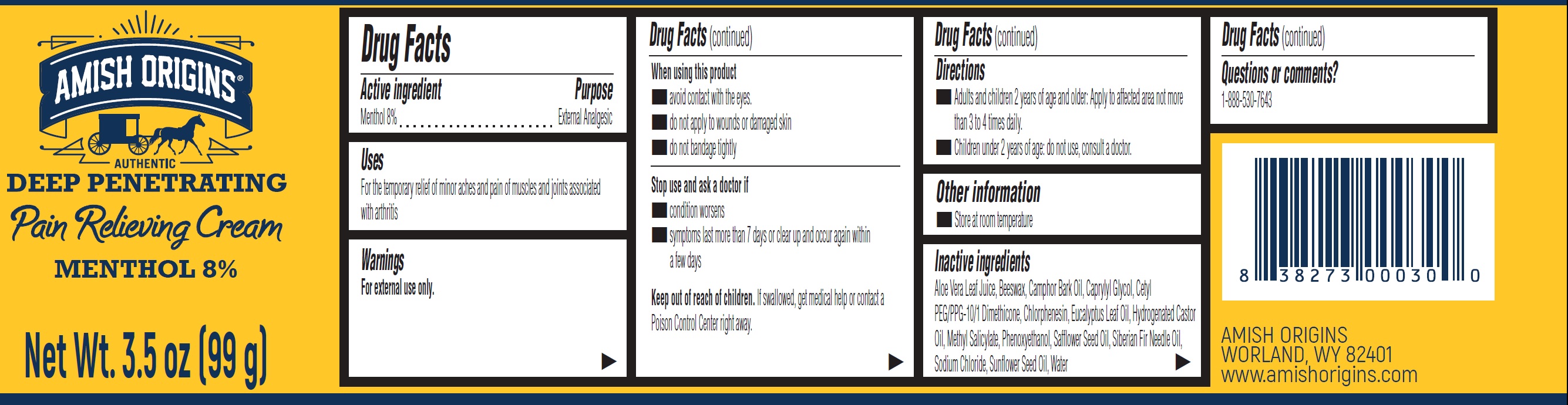
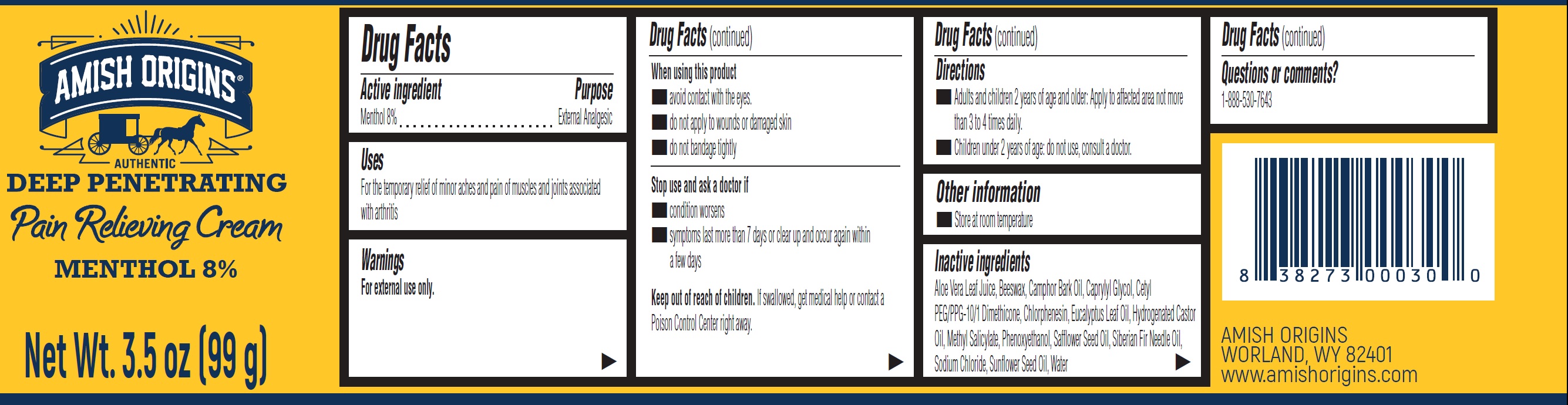
Label: AMISH ORIGINS PAIN RELIEVING MENTHOL 8%- menthol cream
- NDC Code(s): 62212-000-01
- Packager: Amish Origins Management, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 29, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Uses
- Warnings
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
AMISH ORIGINS PAIN RELIEVING MENTHOL 8%
menthol creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62212-000 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 80 mg in 1 g Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) YELLOW WAX (UNII: 2ZA36H0S2V) CAMPHOR OIL (UNII: 75IZZ8Y727) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) EUCALYPTUS OIL (UNII: 2R04ONI662) HYDROGENATED CASTOR OIL (UNII: ZF94AP8MEY) METHYL SALICYLATE (UNII: LAV5U5022Y) PHENOXYETHANOL (UNII: HIE492ZZ3T) SODIUM CHLORIDE (UNII: 451W47IQ8X) SUNFLOWER OIL (UNII: 3W1JG795YI) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62212-000-01 99 g in 1 JAR; Type 0: Not a Combination Product 01/01/2001 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 01/01/2001 Labeler - Amish Origins Management, LLC (079239259)