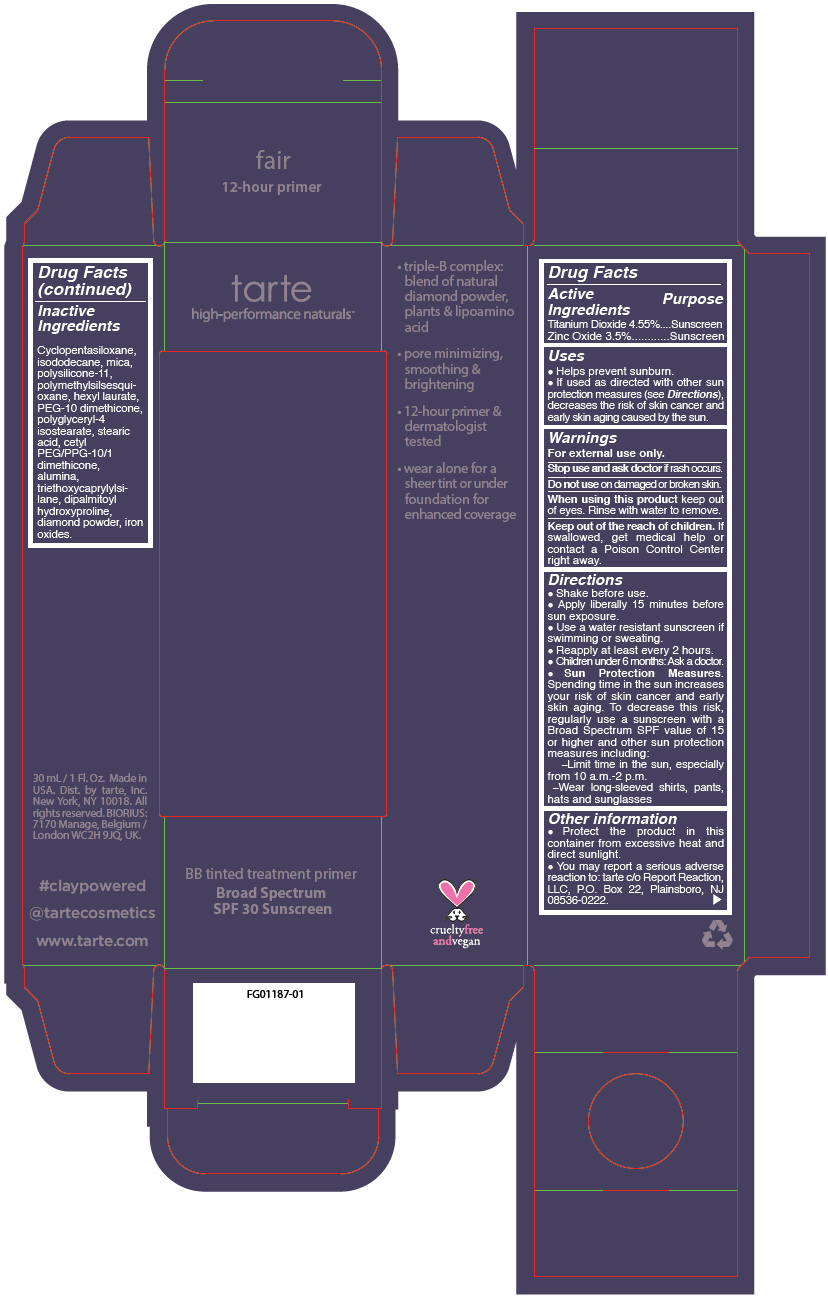
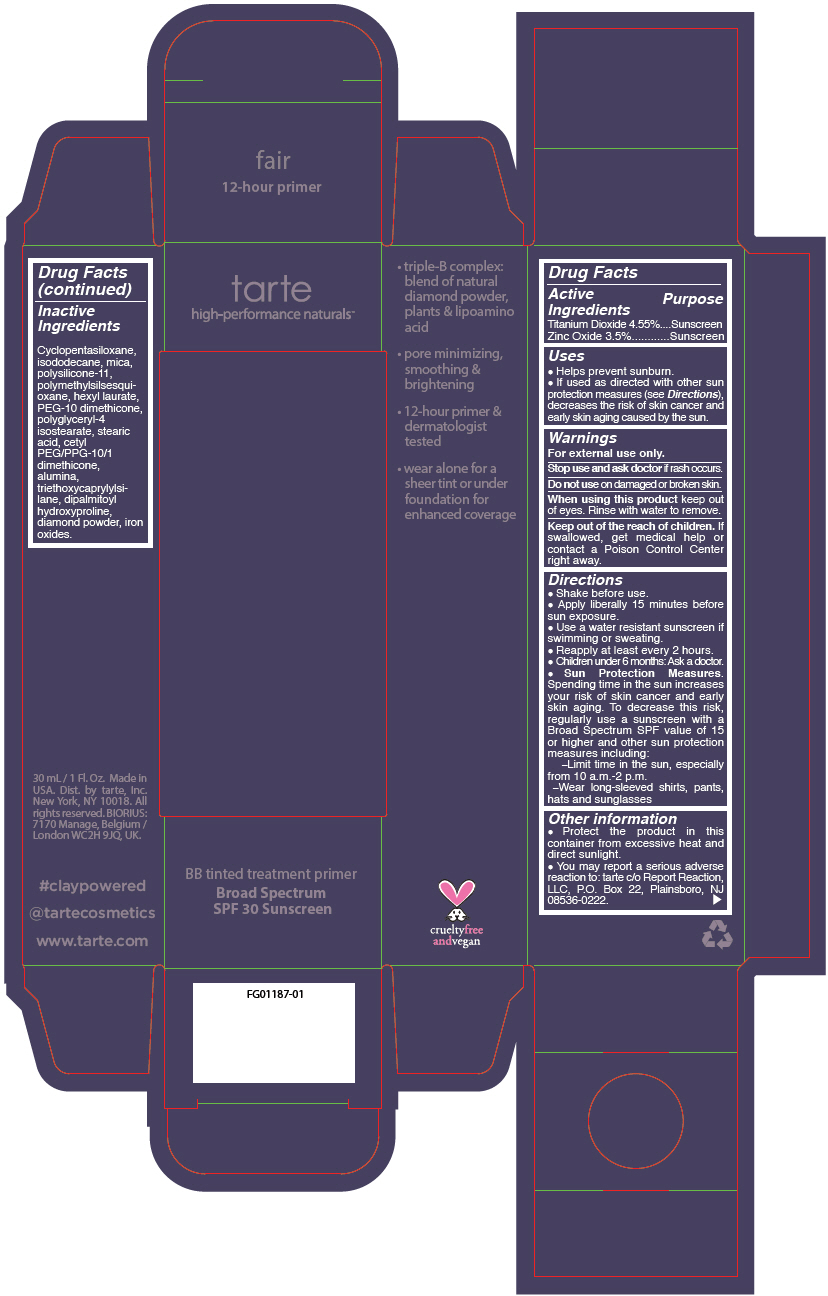
Label: BB TINTED TREATMENT PRIMER BROAD SPECTRUM SPF 30 SUNSCREEN FAIR- titanium dioxide and zinc oxide liquid
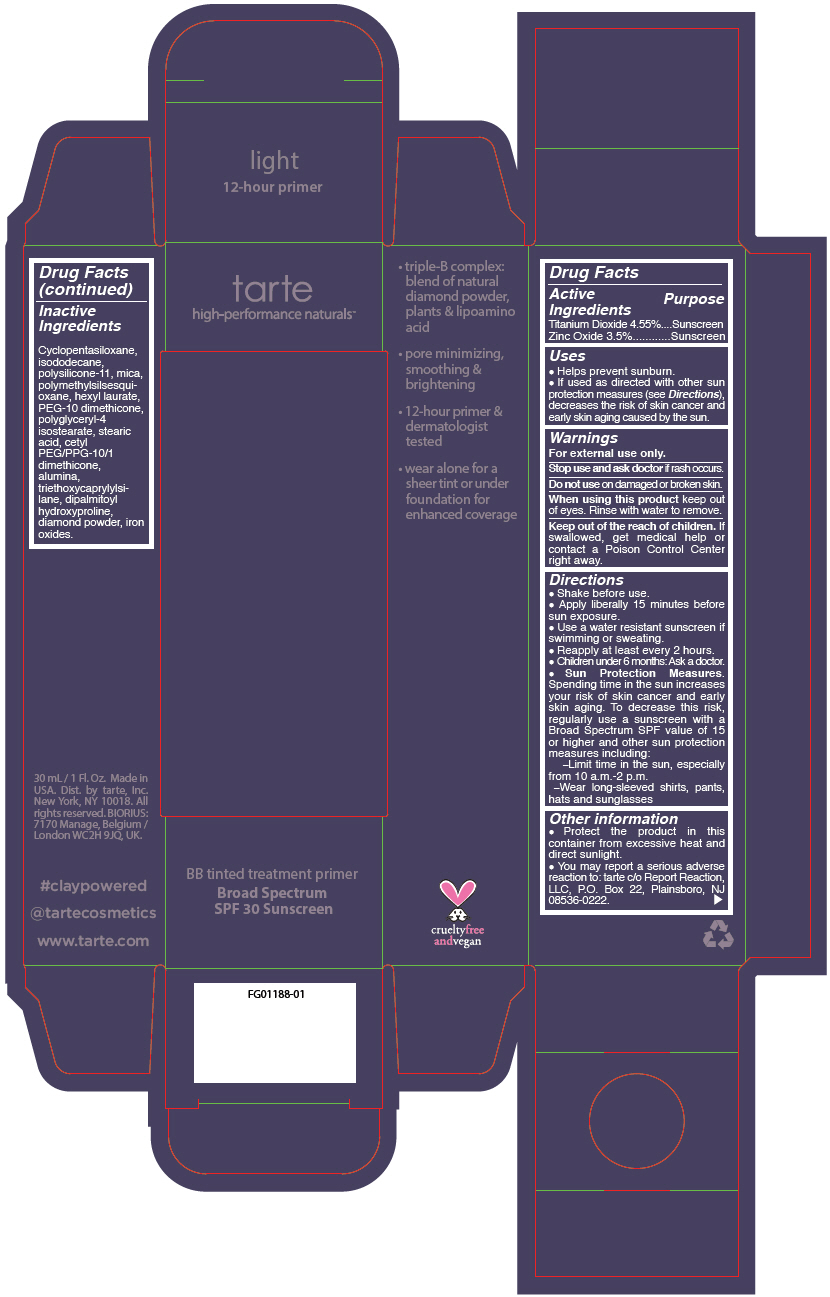
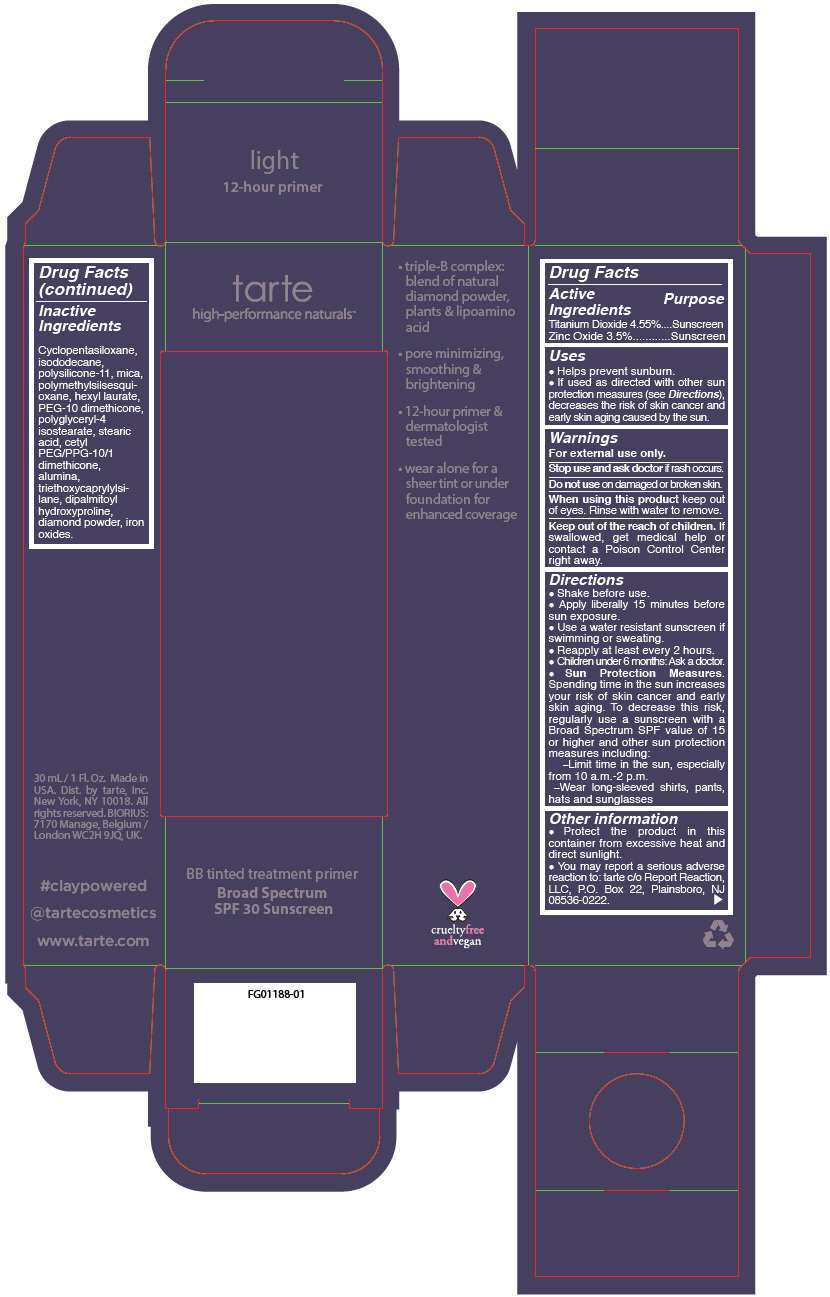
BB TINTED TREATMENT PRIMER BROAD SPECTRUM SPF 30 SUNSCREEN LIGHT- titanium dioxide and zinc oxide liquid
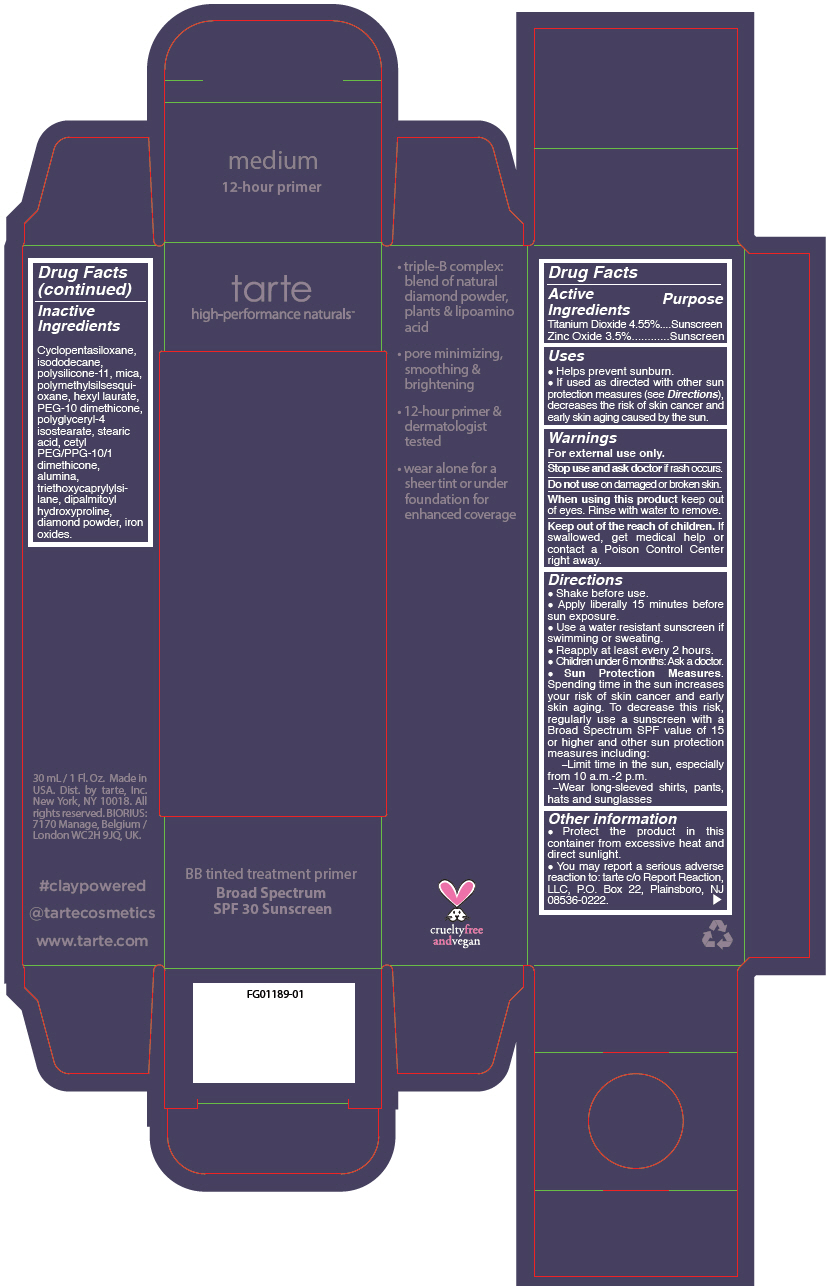
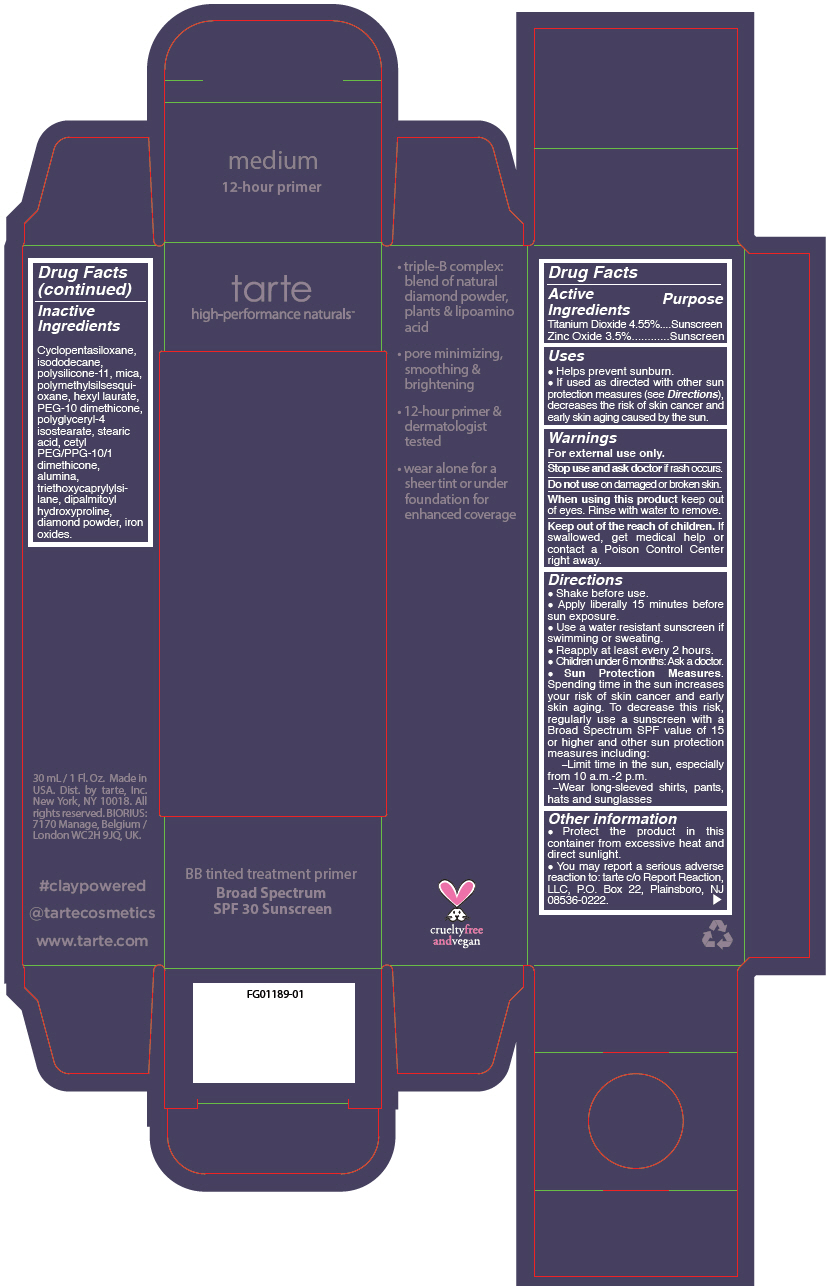
BB TINTED TREATMENT PRIMER BROAD SPECTRUM SPF 30 SUNSCREEN MEDIUM- titanium dioxide and zinc oxide liquid
BB TINTED TREATMENT PRIMER BROAD S .......ium dioxide and zinc oxide) liquid
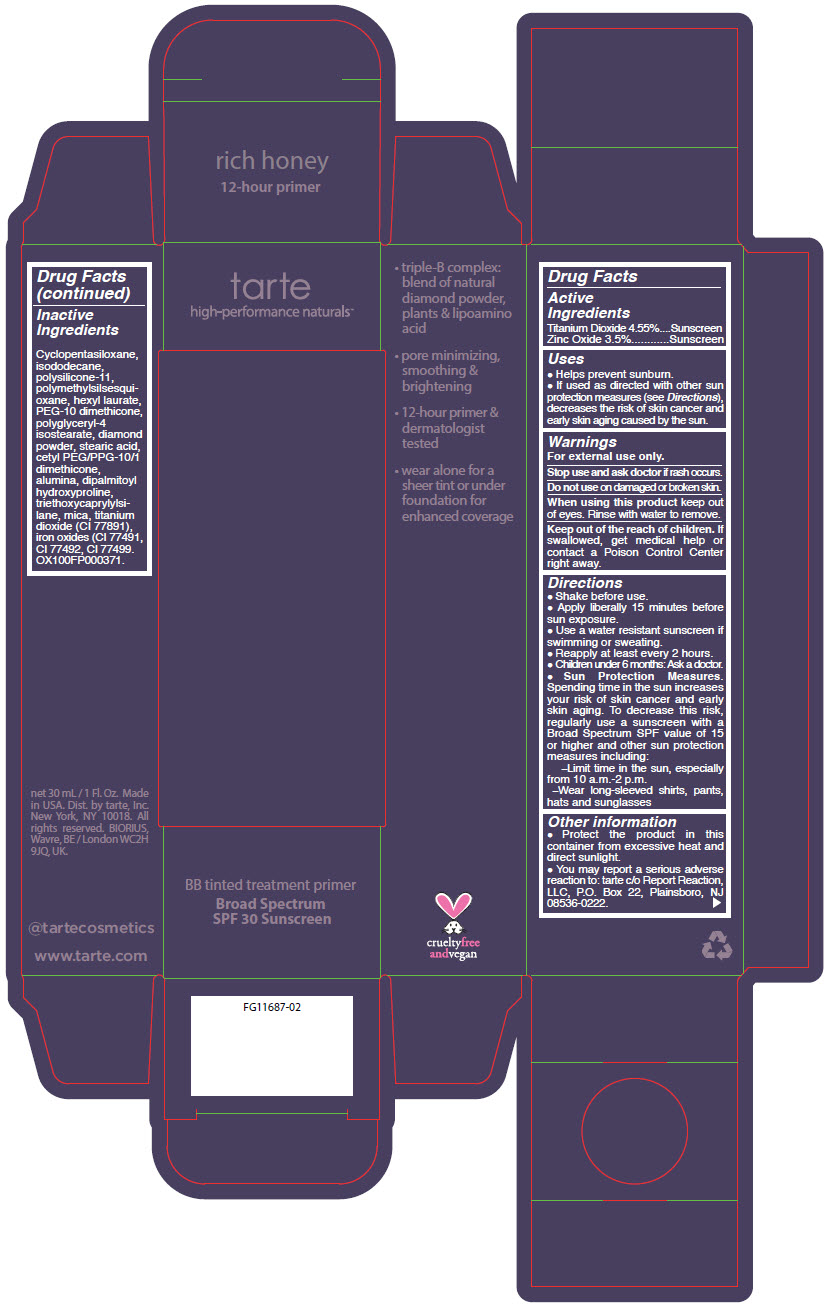
BB TINTED TREATMENT PRIMER BROAD SPECTRUM SPF 30 SUNSCREEN RICH HONEY- titanium dioxide and zinc oxide liquid
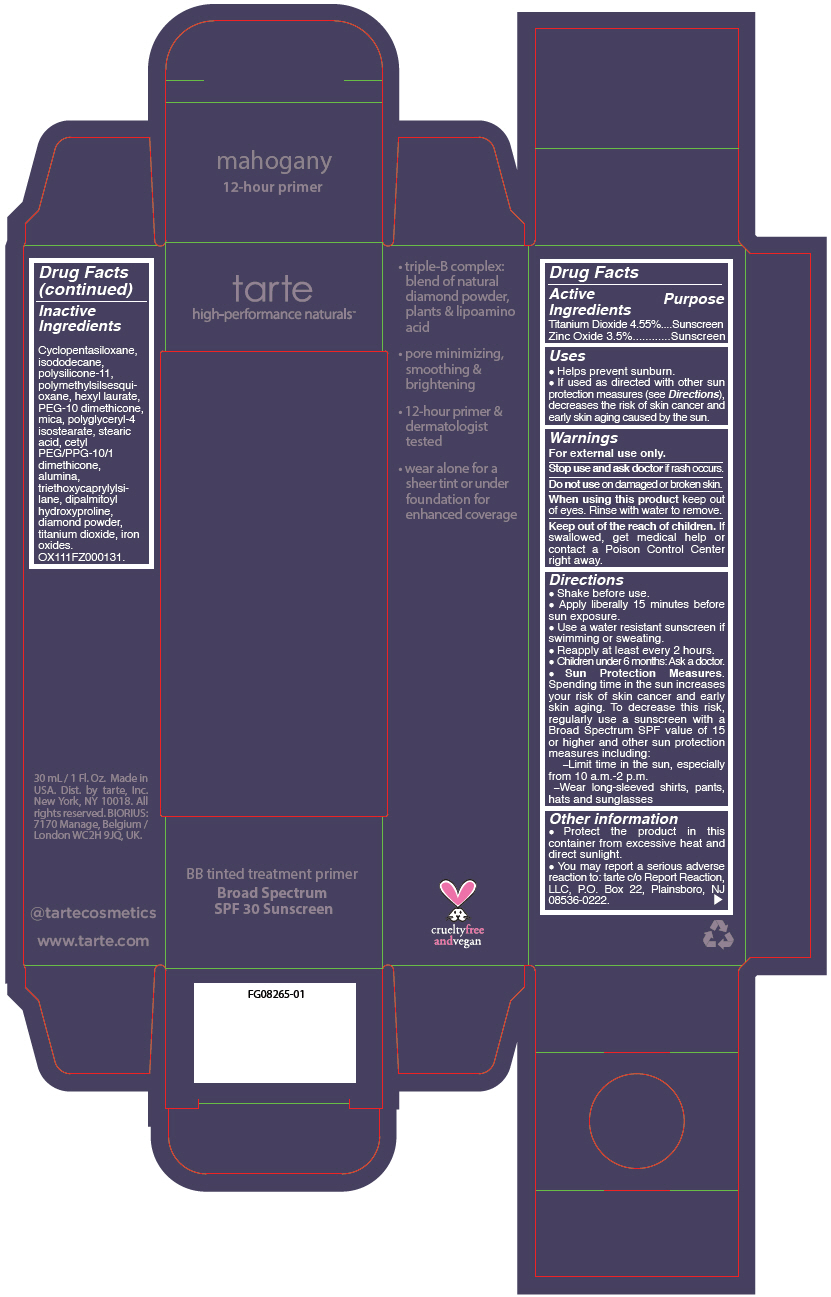
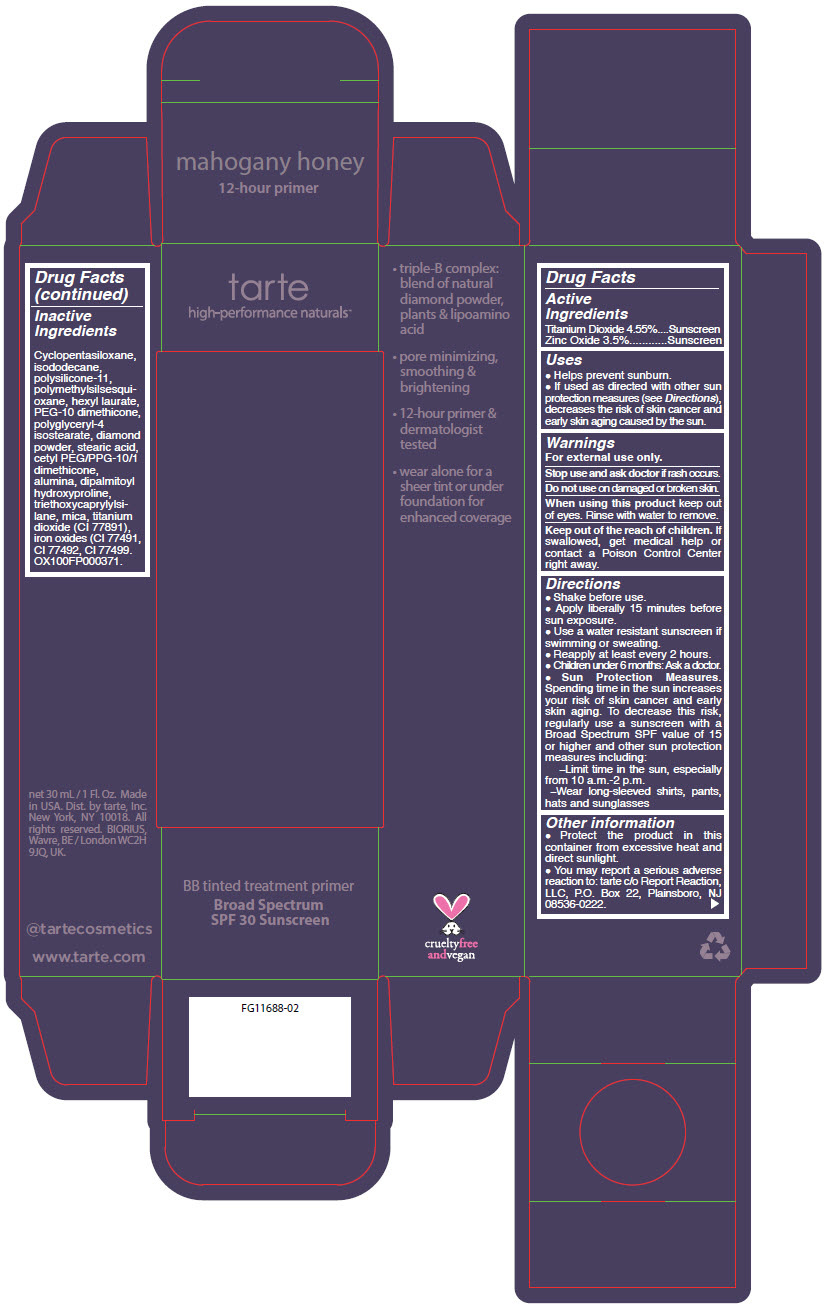
BB TINTED TREATMENT PRIMER BROAD SPECTRUM SPF 30 SUNSCREEN MAHOGANY HONEY- titanium dioxide and zinc oxide liquid
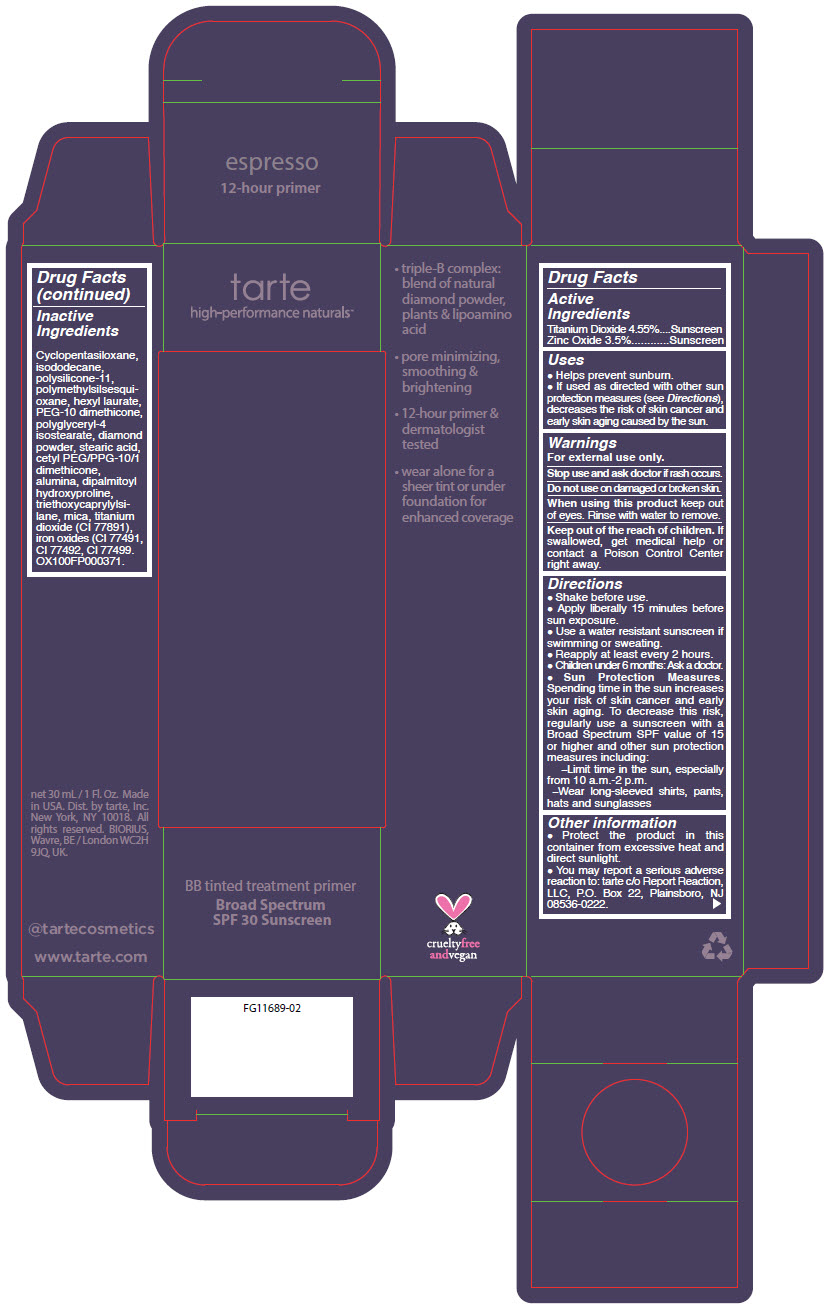
BB TINTED TREATMENT PRIMER BROAD SPECTRUM SPF 30 SUNSCREEN ESPRESSO- titanium dioxide and zinc oxide liquid
-
NDC Code(s):
51060-243-01,
51060-244-01,
51060-245-01,
51060-246-01, view more51060-247-01, 51060-248-01, 51060-249-01, 51060-250-01, 51060-251-01, 51060-359-01, 51060-360-01, 51060-361-01, 51060-362-01, 51060-363-01, 51060-364-01
- Packager: Tarte, Inc.
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated August 4, 2022
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
-
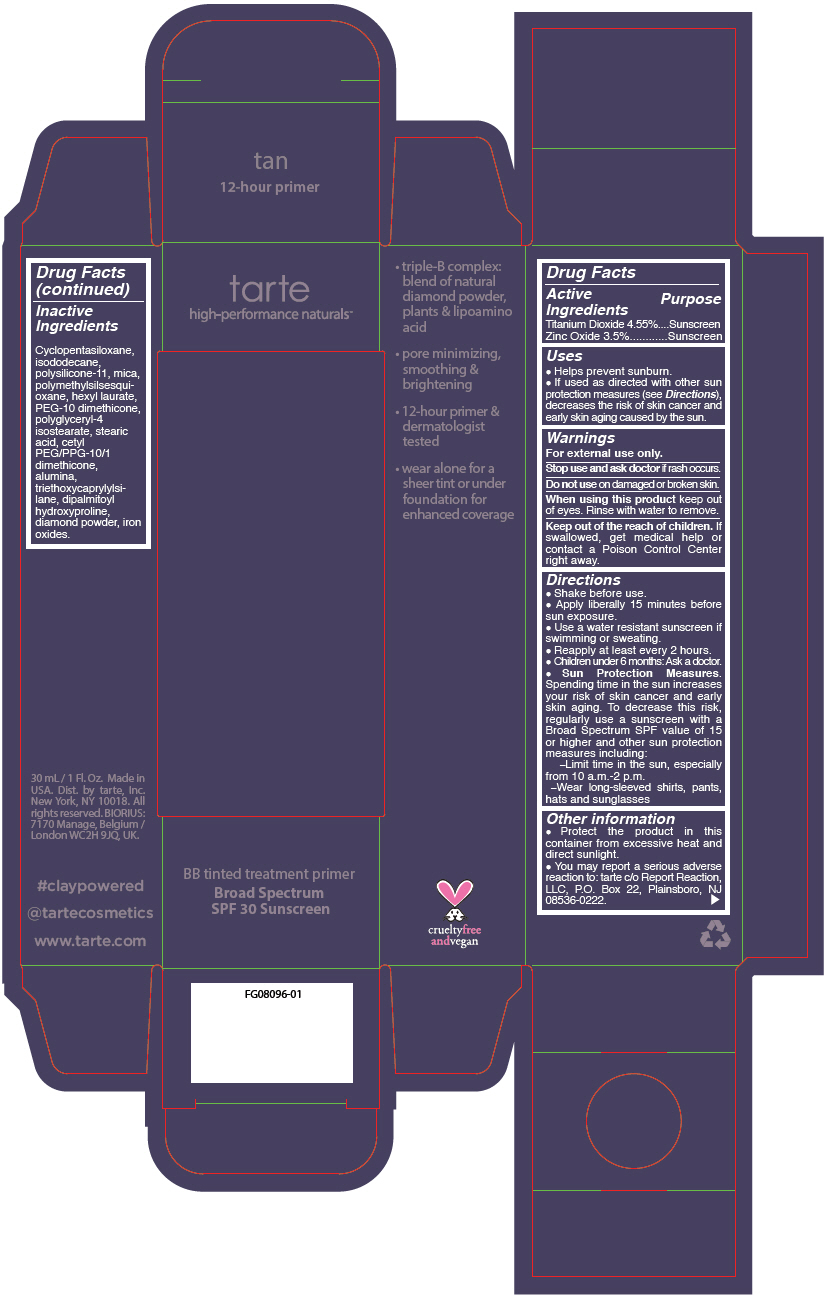
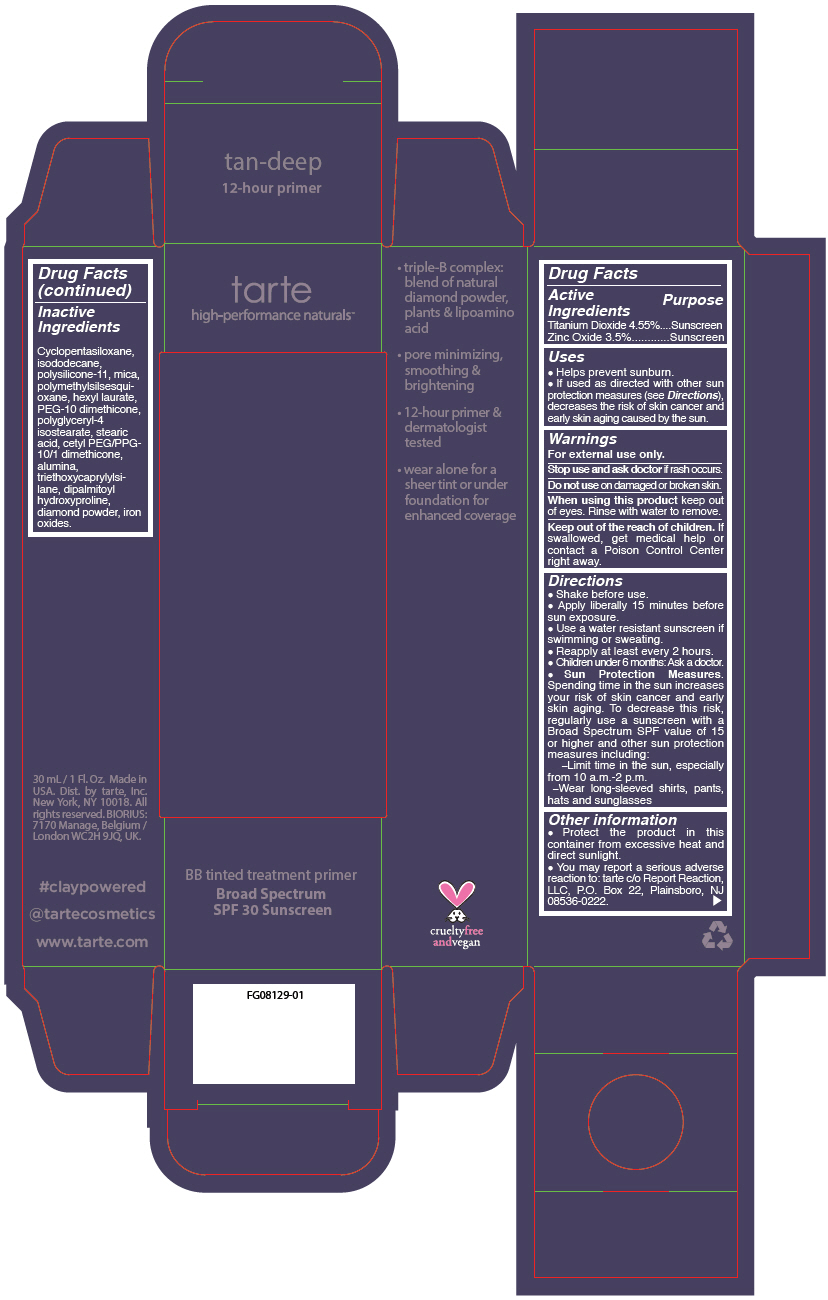
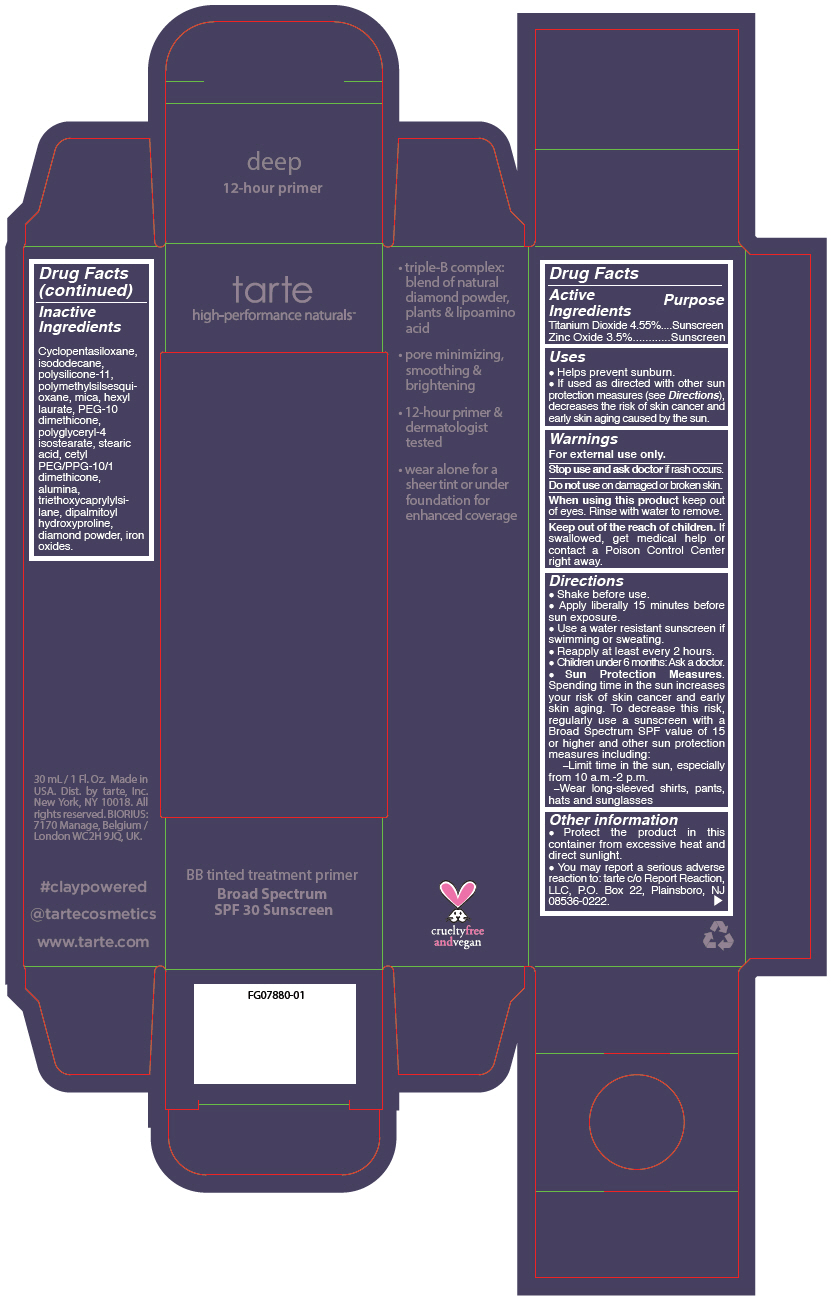
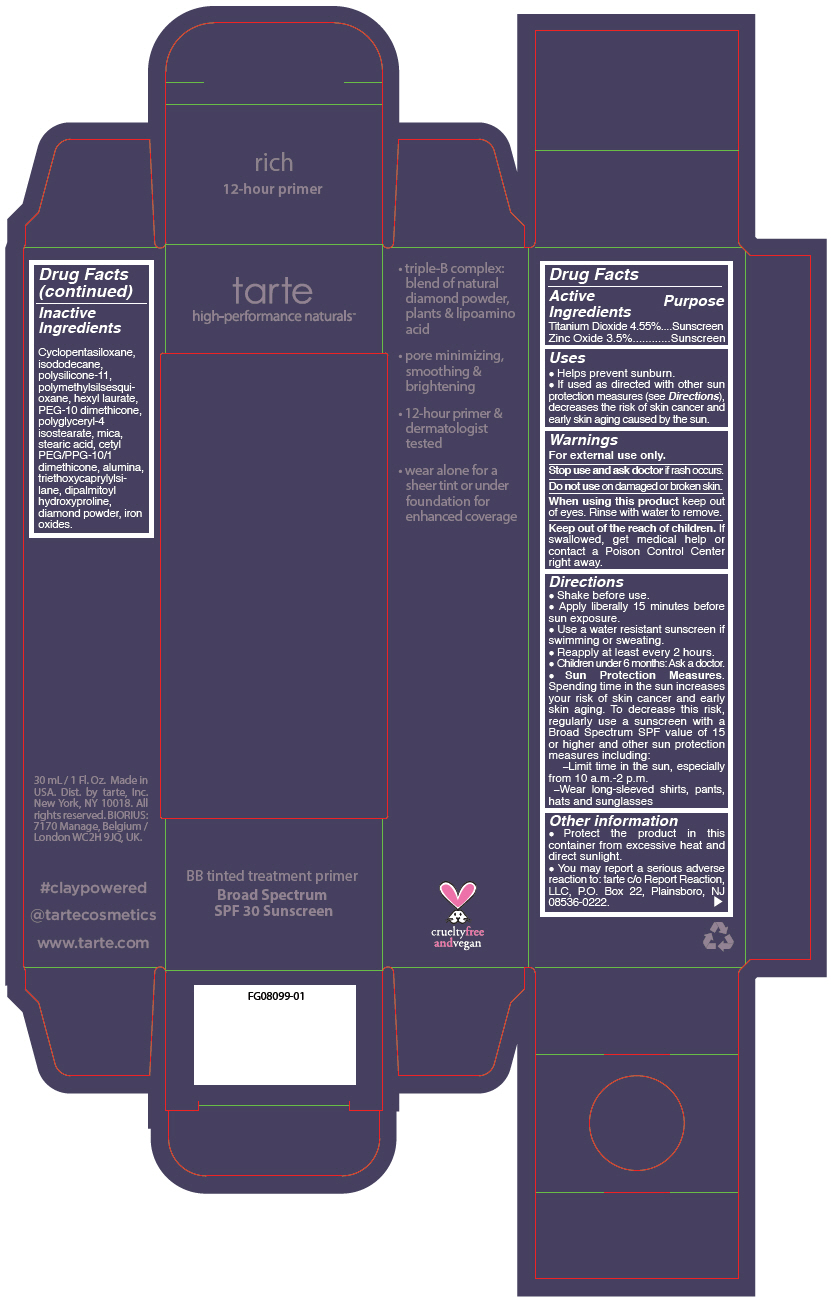
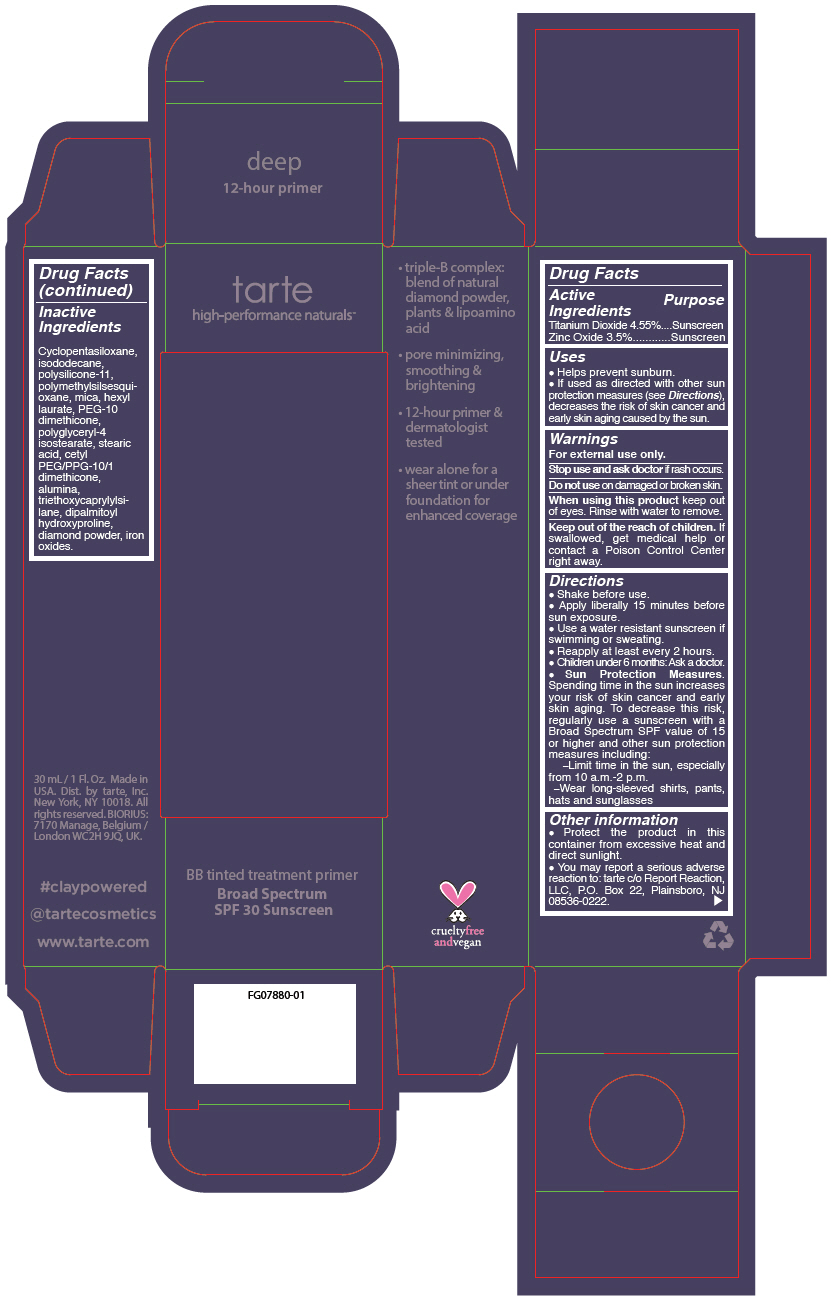
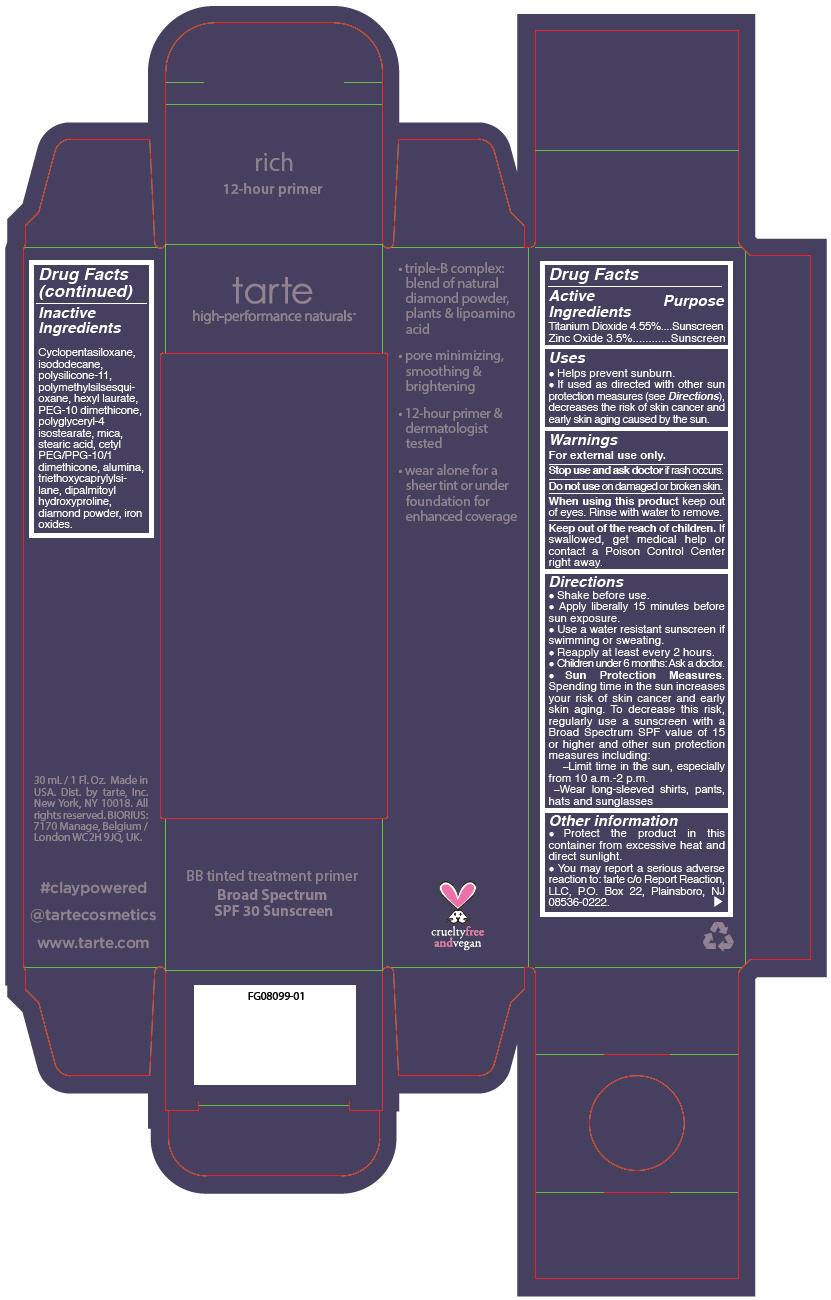
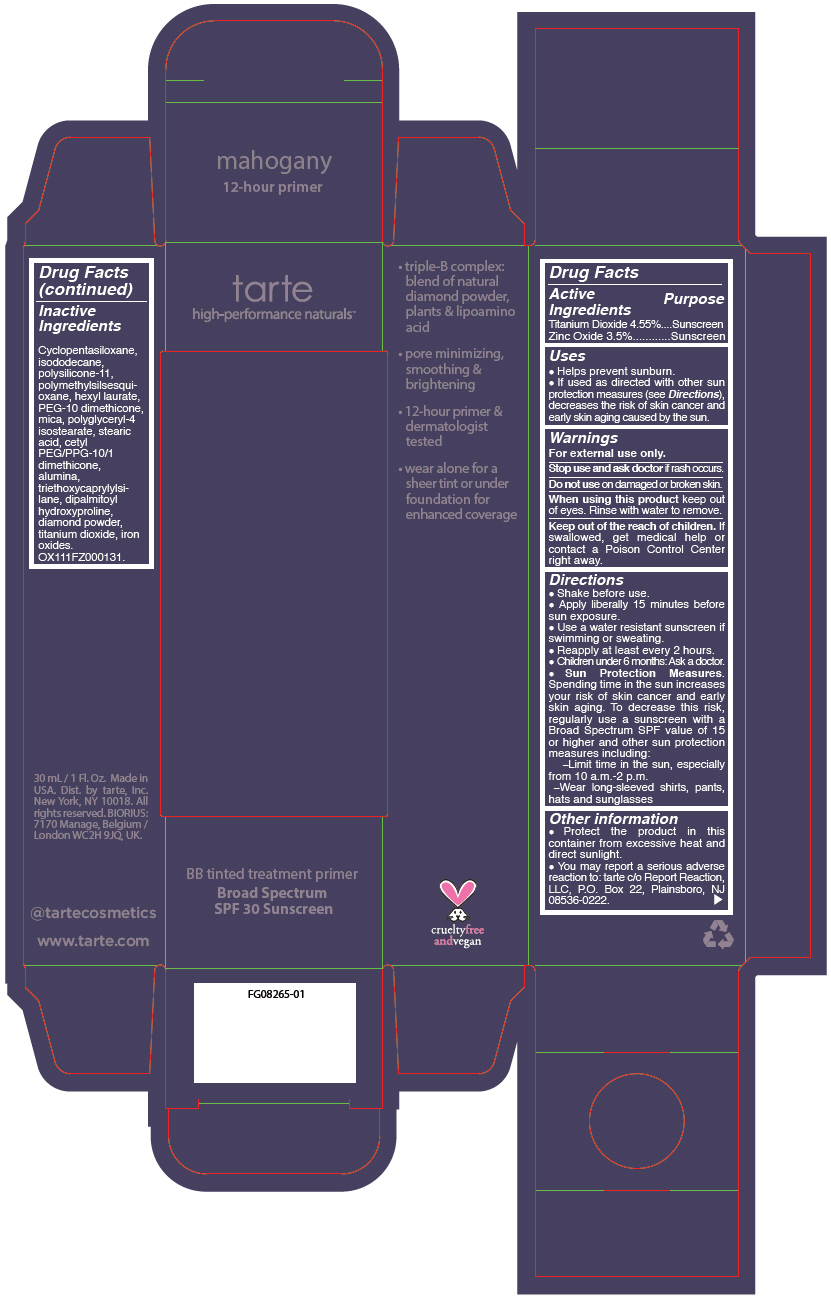
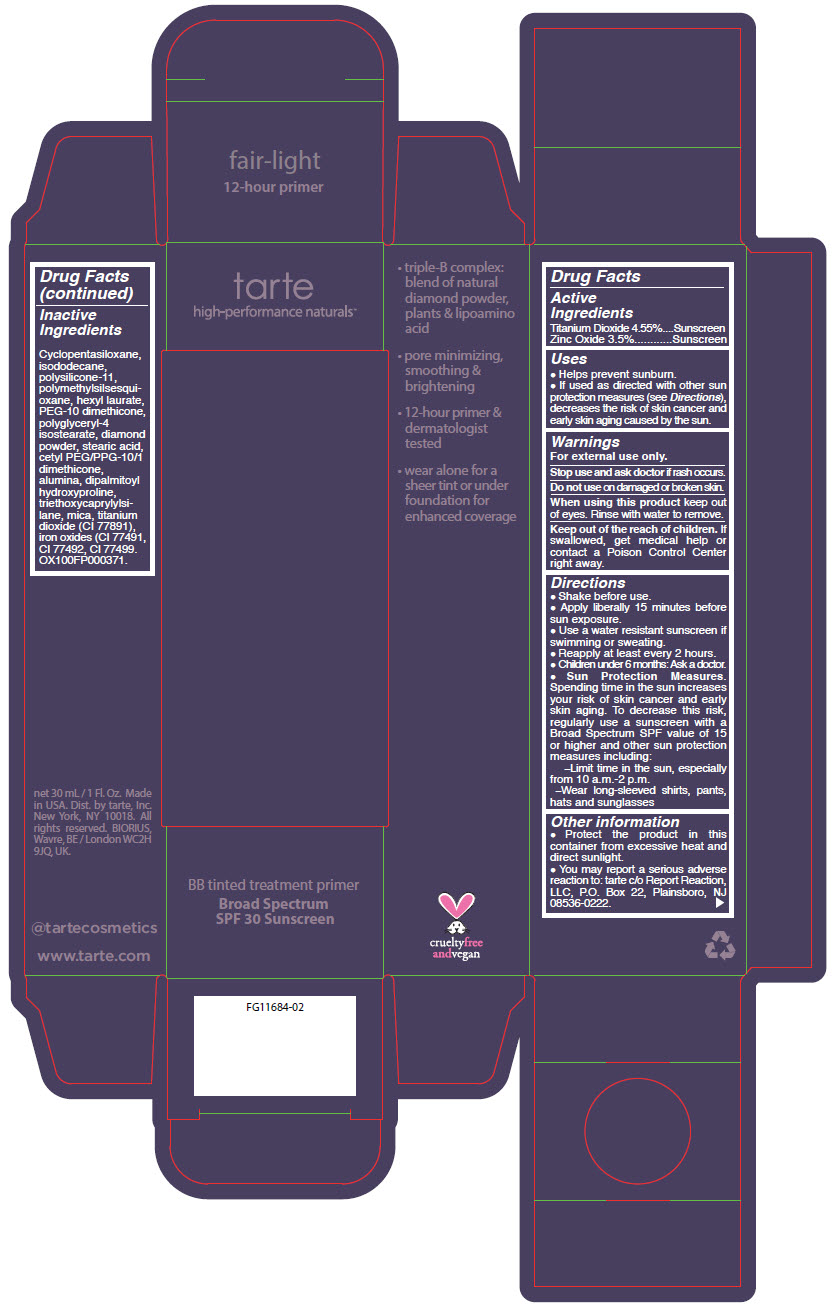
Uses
- Helps prevent sunburn.
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
- Warnings
-
Directions
- Shake before use.
- Apply liberally 15 minutes before sun exposure.
- Use a water resistant sunscreen if swimming or sweating.
- Reapply at least every 2 hours.
- Children under 6 months: Ask a doctor.
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- –
- Limit time in the sun, especially from 10 a.m.-2 p.m.
- –
- Wear long-sleeved shirts, pants, hats and sunglasses
- Other information
- Inactive Ingredients
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 30 mL Tube Carton - fair
- PRINCIPAL DISPLAY PANEL - 30 mL Tube Carton - light
- PRINCIPAL DISPLAY PANEL - 30 mL Tube Carton - medium
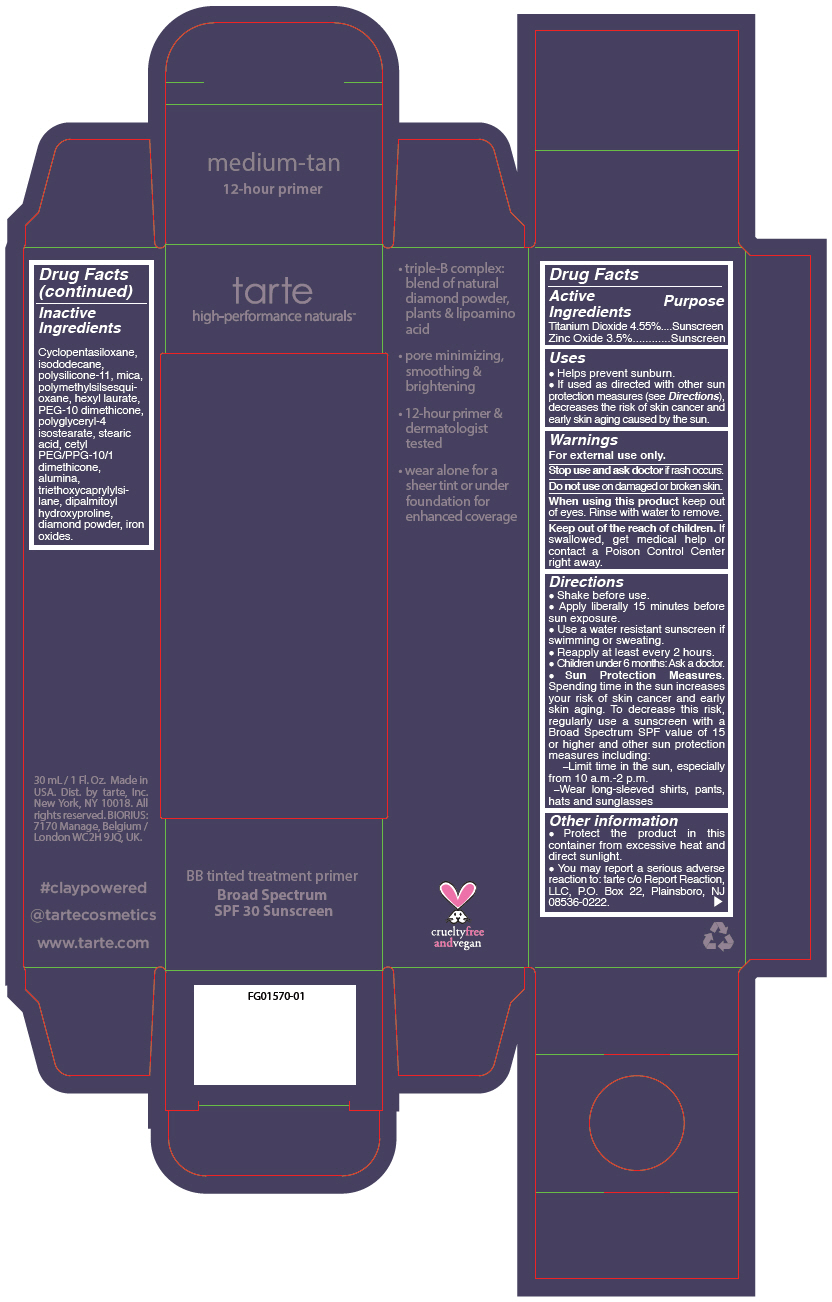
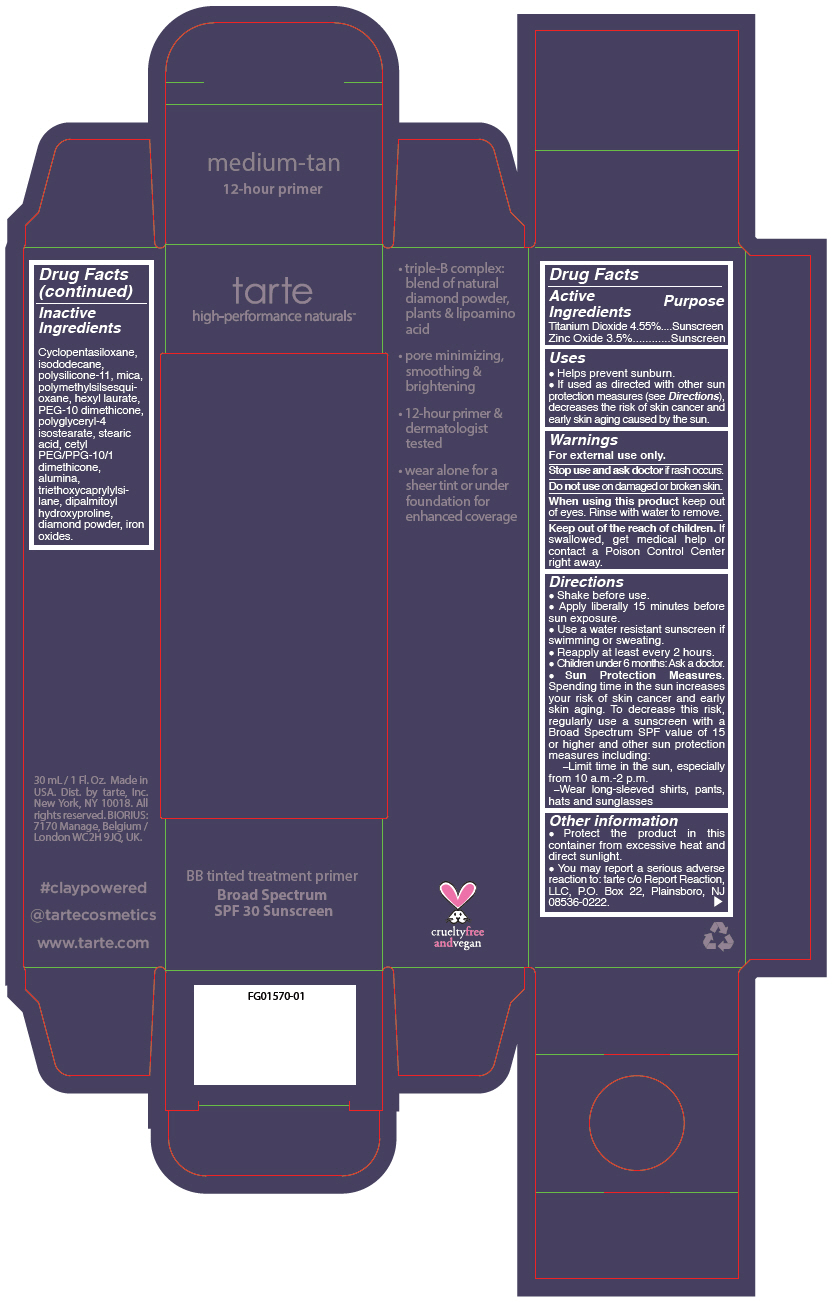
- PRINCIPAL DISPLAY PANEL - 30 mL Tube Carton - medium-tan
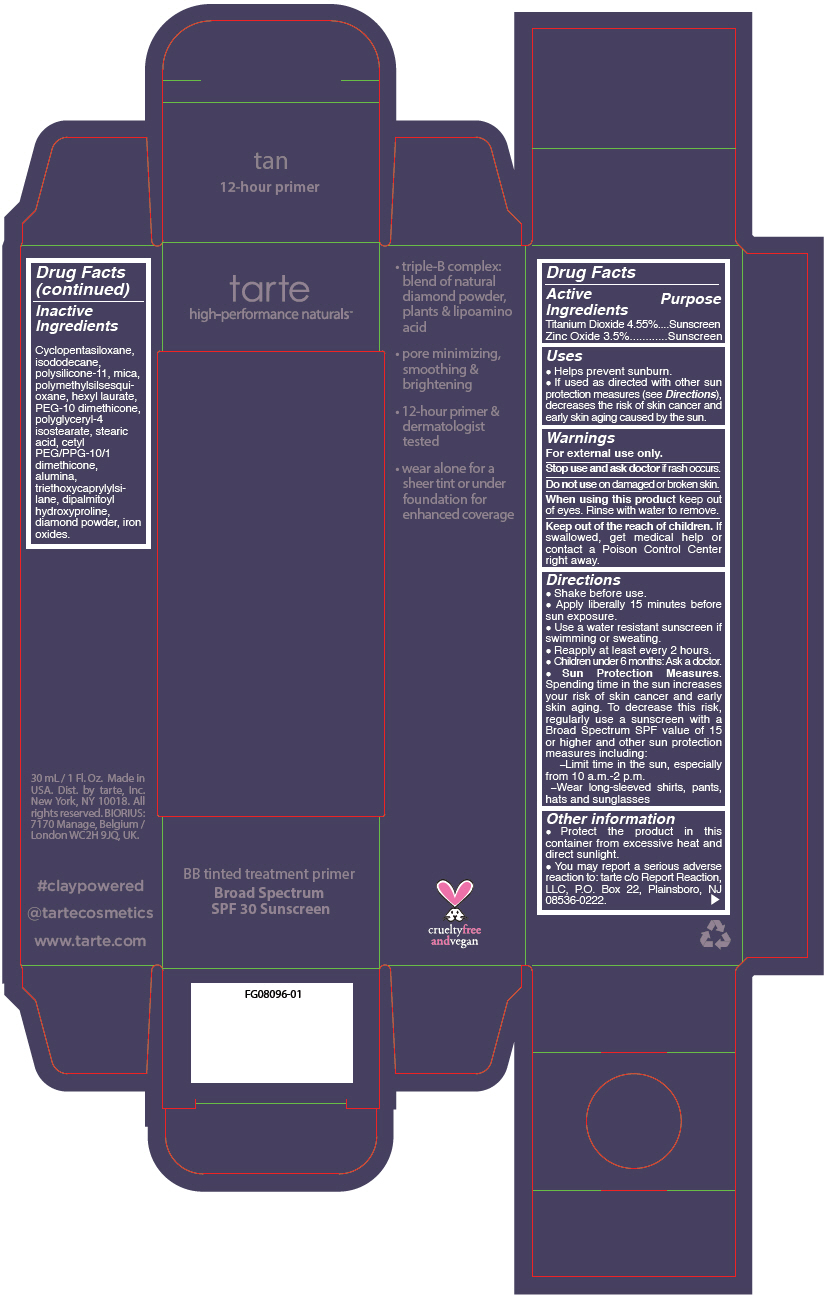
- PRINCIPAL DISPLAY PANEL - 30 mL Tube Carton - tan
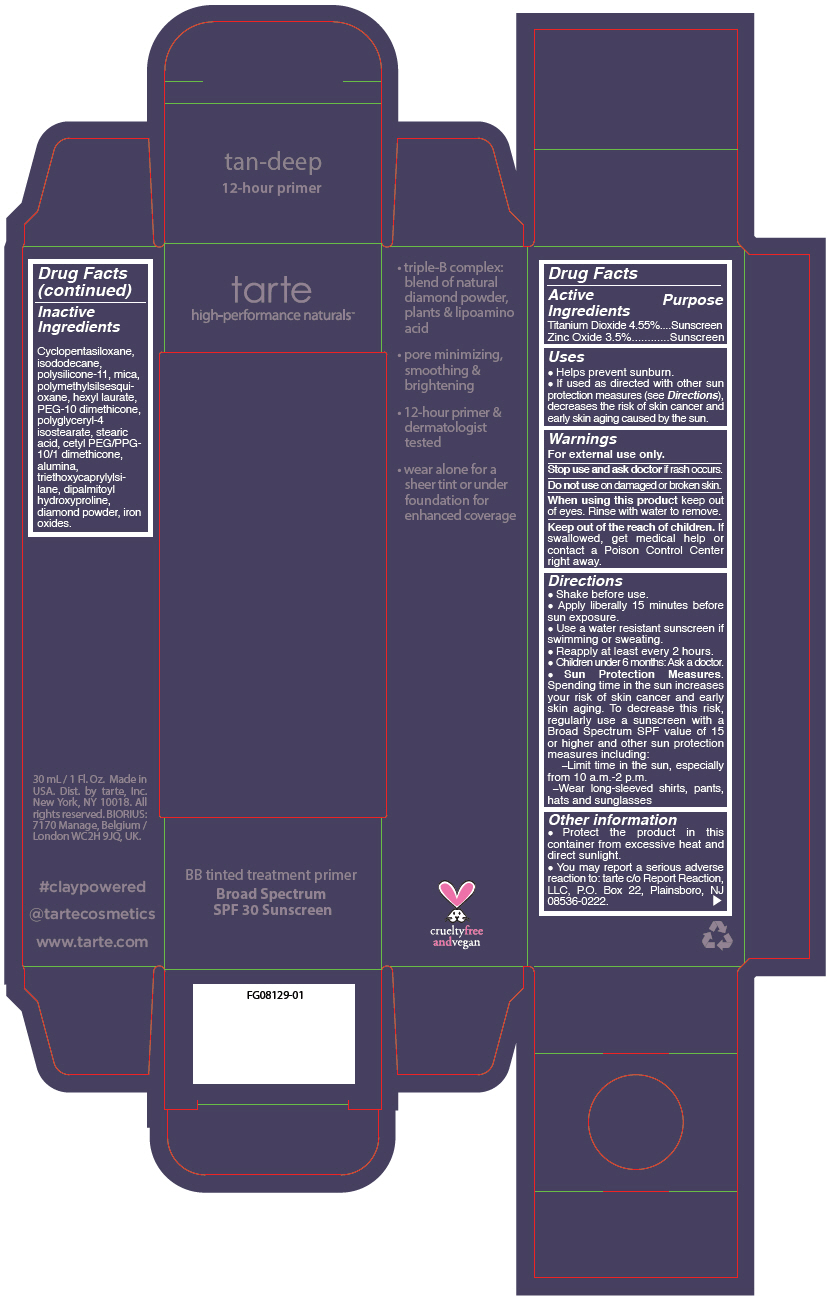
- PRINCIPAL DISPLAY PANEL - 30 mL Tube Carton - tan-deep
- PRINCIPAL DISPLAY PANEL - 30 mL Tube Carton - deep
- PRINCIPAL DISPLAY PANEL - 30 mL Tube Carton - rich
- PRINCIPAL DISPLAY PANEL - 30 mL Tube Carton - mahogany
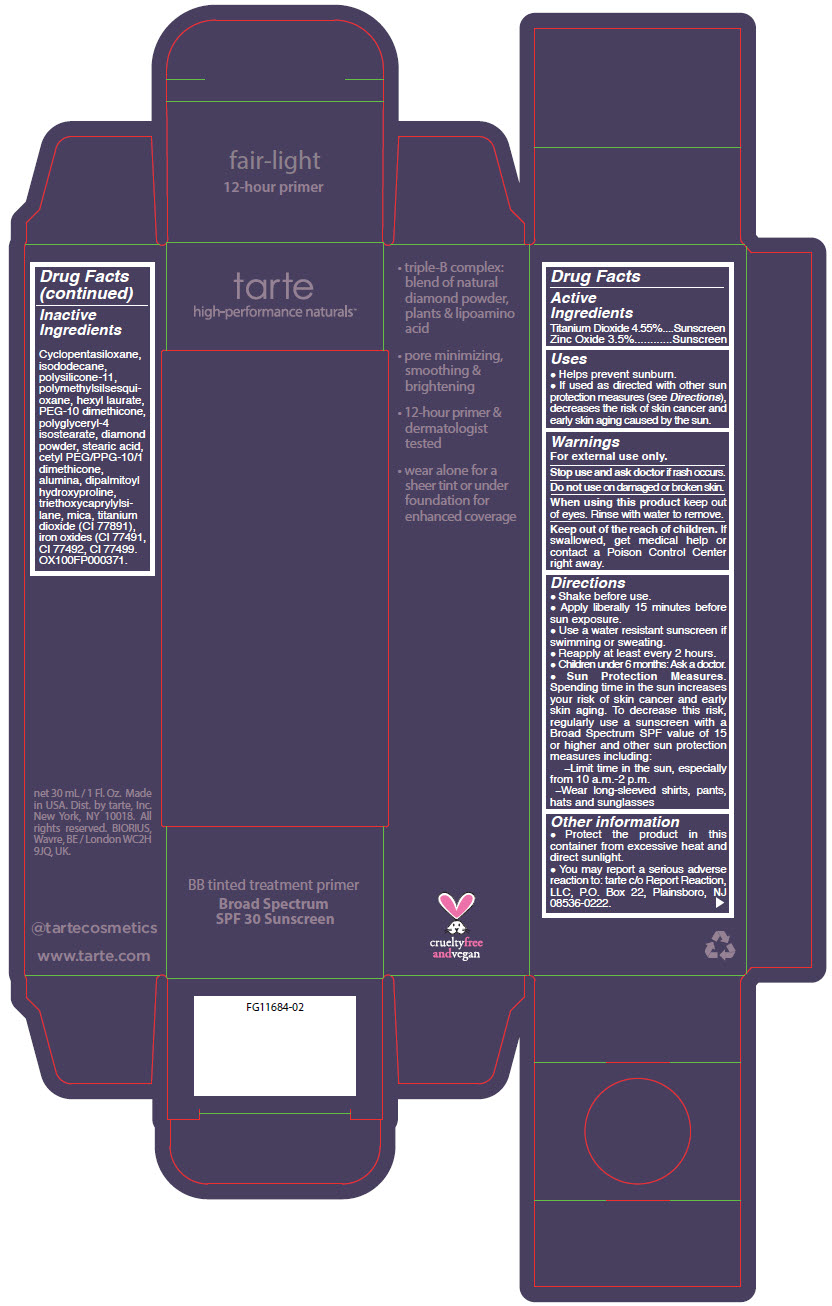
- PRINCIPAL DISPLAY PANEL - 30 mL Tube Carton - fair-light
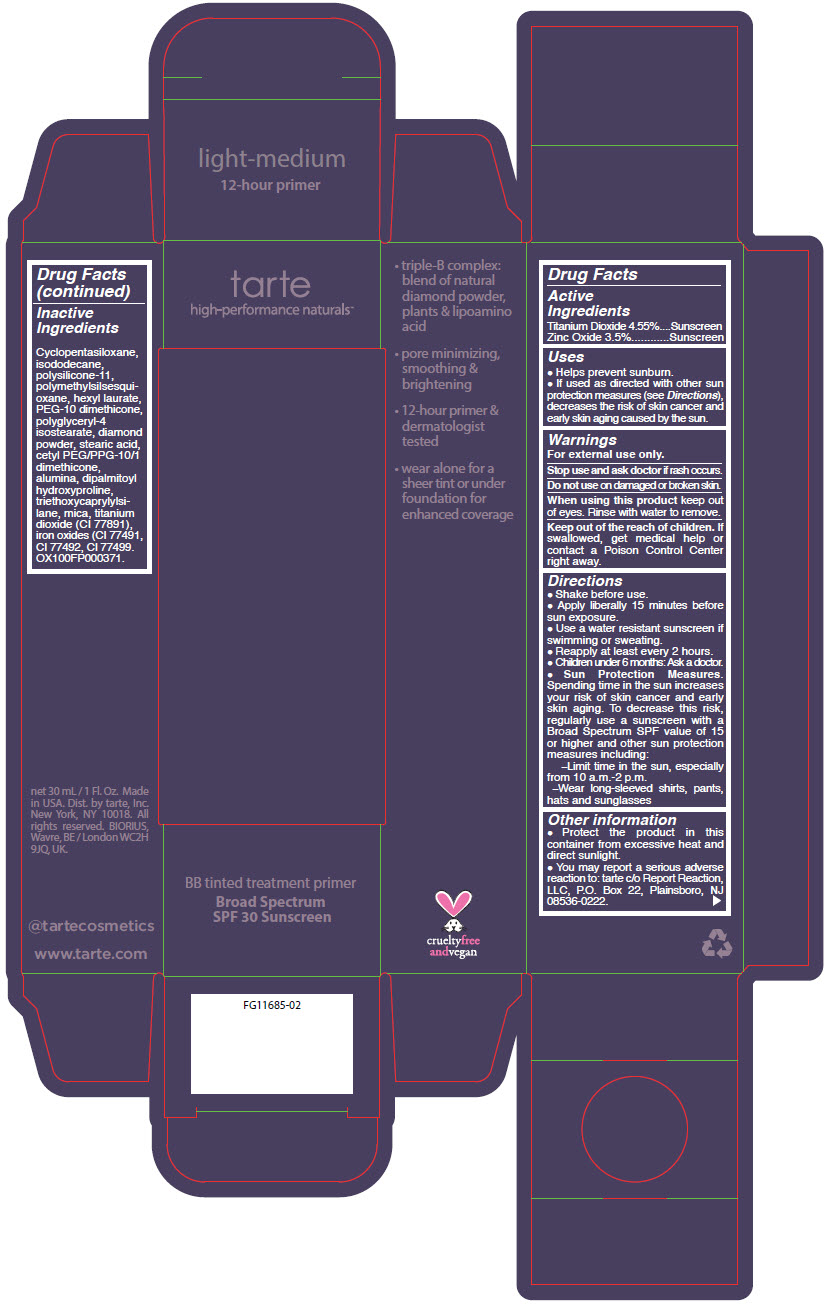
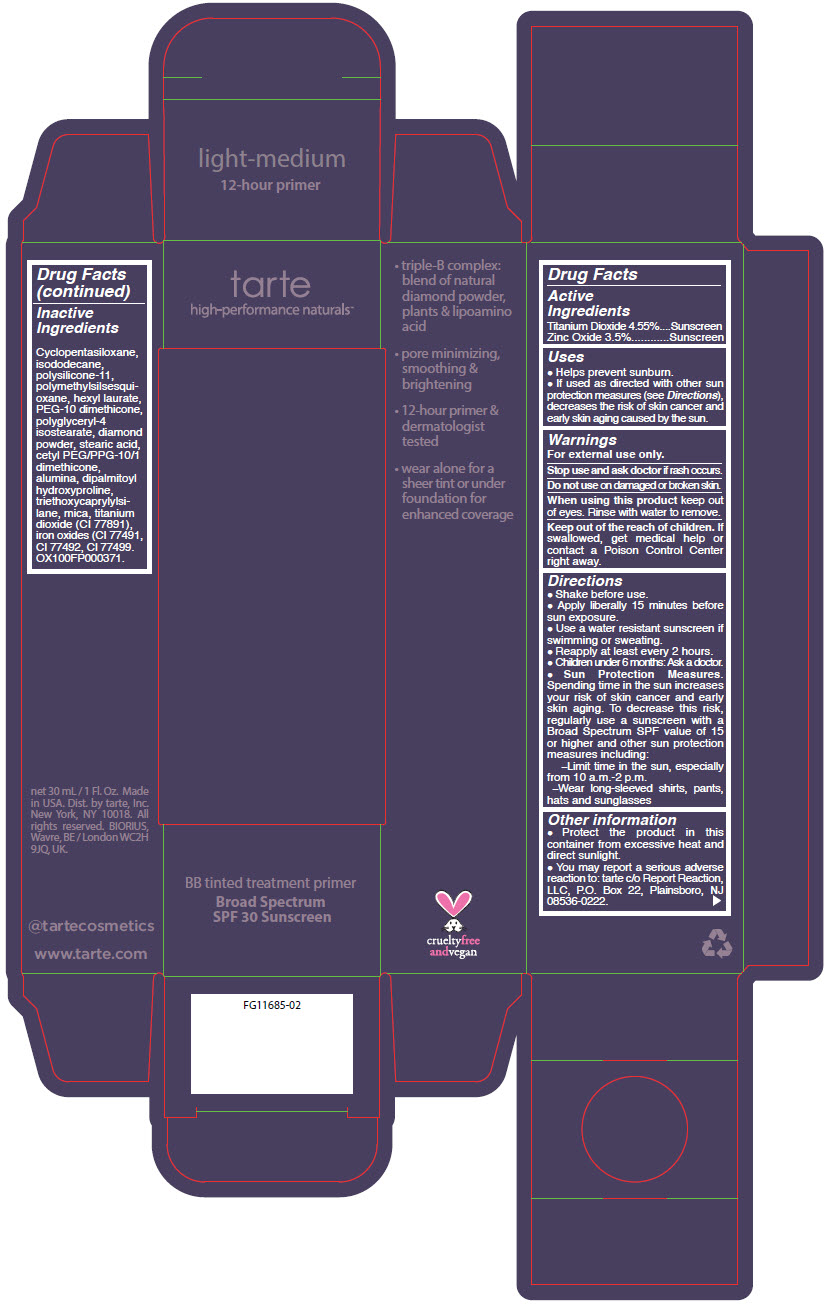
- PRINCIPAL DISPLAY PANEL - 30 mL Tube Carton - light-medium
- PRINCIPAL DISPLAY PANEL - 30 mL Tube Carton - deep sand
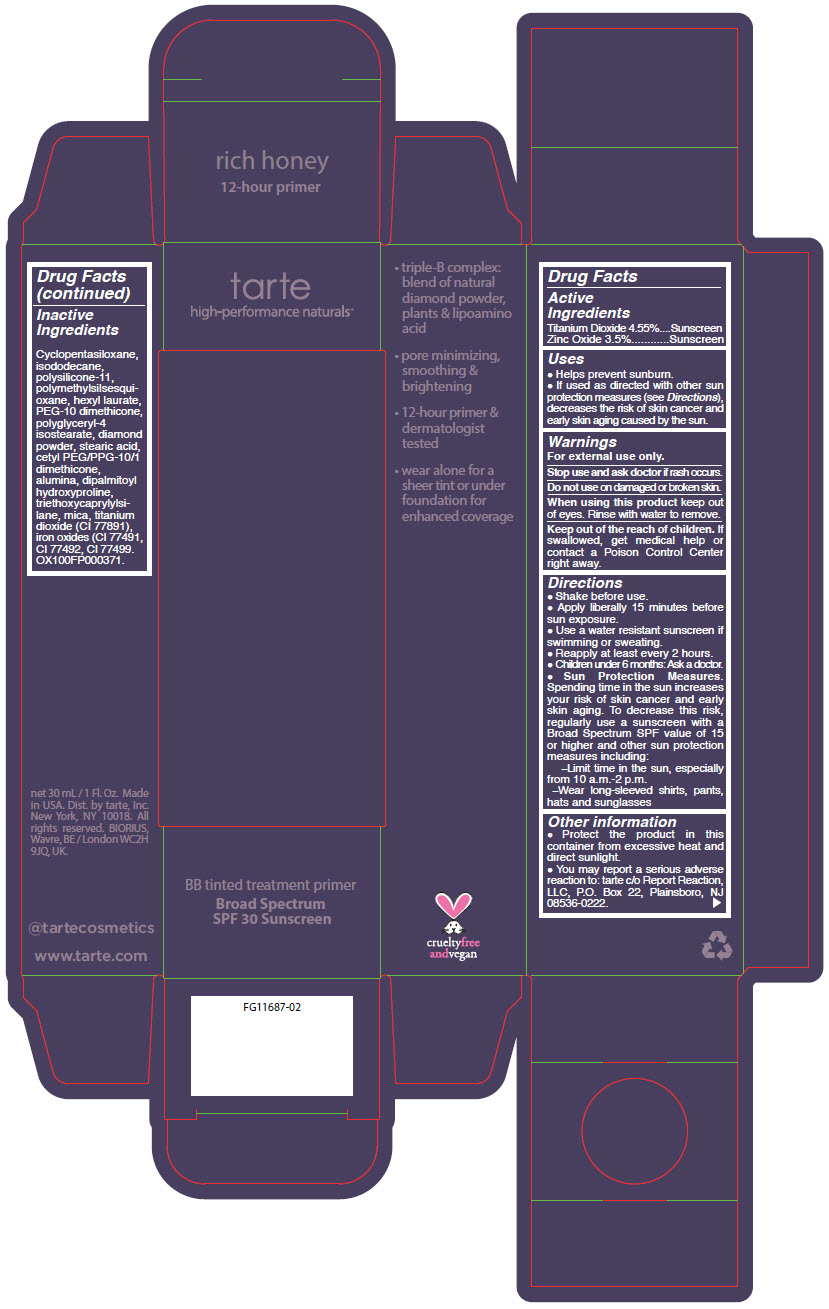
- PRINCIPAL DISPLAY PANEL - 30 mL Tube Carton - rich honey
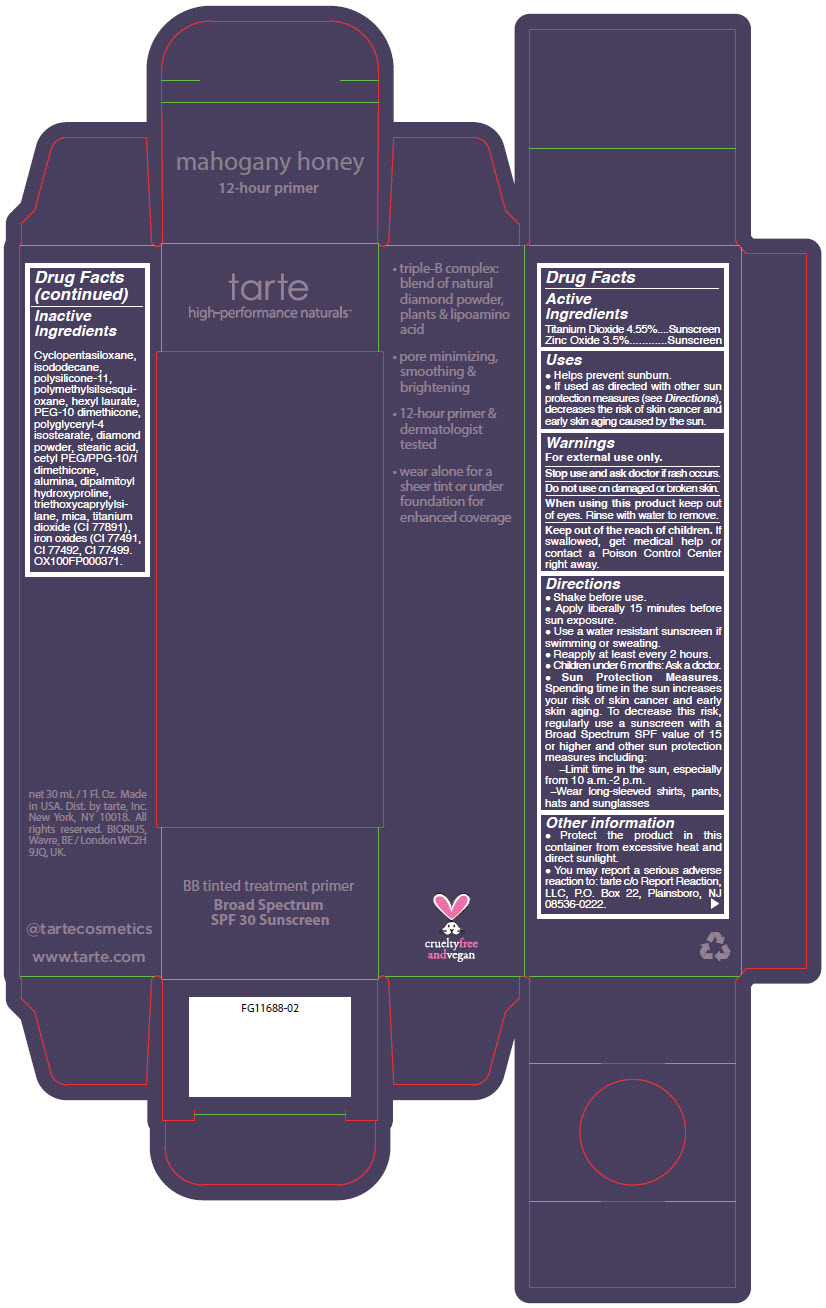
- PRINCIPAL DISPLAY PANEL - 30 mL Tube Carton - mahogany honey
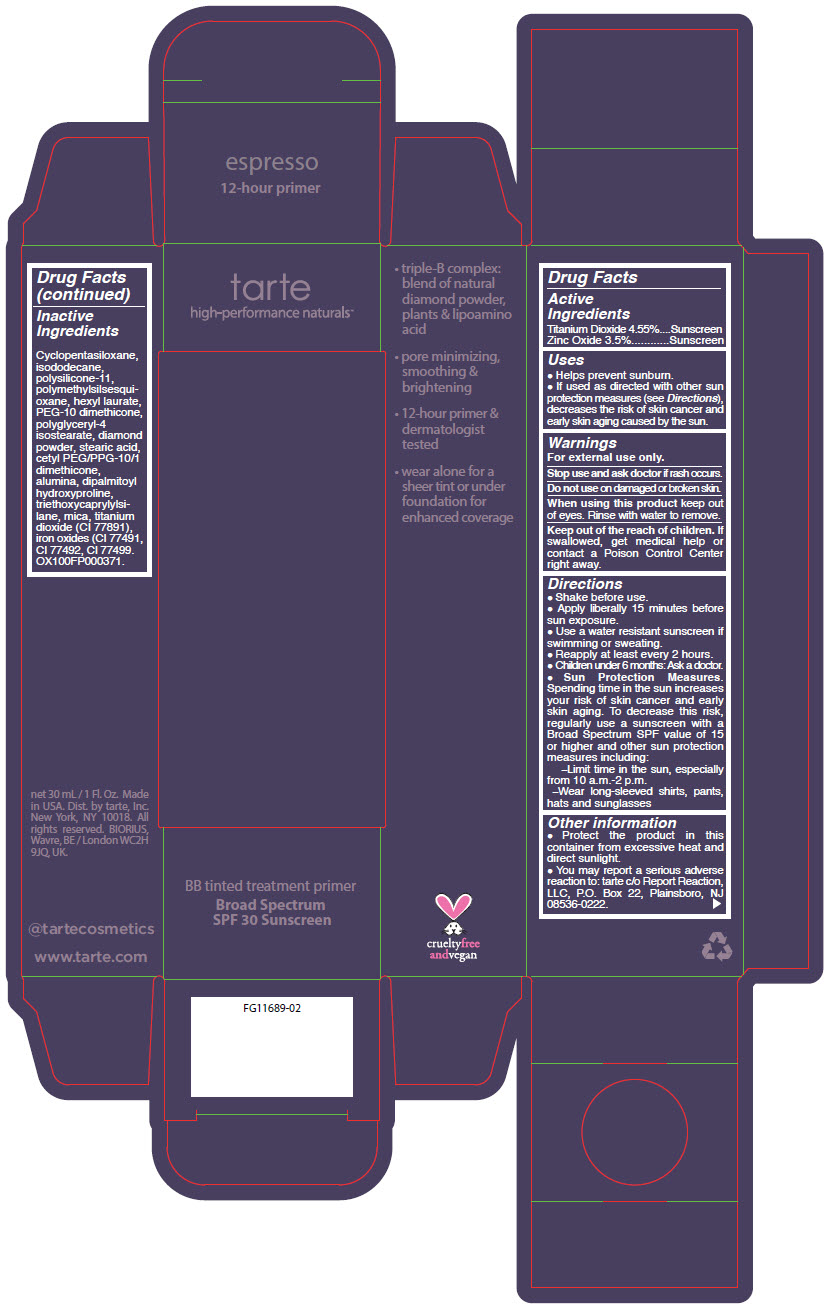
- PRINCIPAL DISPLAY PANEL - 30 mL Tube Carton - espresso
-
INGREDIENTS AND APPEARANCE
BB TINTED TREATMENT PRIMER BROAD SPECTRUM SPF 30 SUNSCREEN FAIR
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-243 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 45.5 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 35 mg in 1 mL Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) ISODODECANE (UNII: A8289P68Y2) MICA (UNII: V8A1AW0880) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) POLYMETHYLSILSESQUIOXANE (11 MICRONS) (UNII: Z570VEV8XK) HEXYL LAURATE (UNII: 4CG9F9W01Q) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) BENZIMIDAZOLE (UNII: E24GX49LD8) DIAMOND (UNII: 6GRV67N0U2) STEARIC ACID (UNII: 4ELV7Z65AP) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) ALUMINUM OXIDE (UNII: LMI26O6933) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) DIPALMITOYL HYDROXYPROLINE (UNII: E6AHA53N1H) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-243-01 1 in 1 CARTON 09/01/2012 1 30 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 09/01/2012 BB TINTED TREATMENT PRIMER BROAD SPECTRUM SPF 30 SUNSCREEN LIGHT
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-244 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 45.5 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 35 mg in 1 mL Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) MICA (UNII: V8A1AW0880) ISODODECANE (UNII: A8289P68Y2) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) POLYMETHYLSILSESQUIOXANE (11 MICRONS) (UNII: Z570VEV8XK) HEXYL LAURATE (UNII: 4CG9F9W01Q) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) BENZIMIDAZOLE (UNII: E24GX49LD8) DIAMOND (UNII: 6GRV67N0U2) STEARIC ACID (UNII: 4ELV7Z65AP) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) ALUMINUM OXIDE (UNII: LMI26O6933) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) DIPALMITOYL HYDROXYPROLINE (UNII: E6AHA53N1H) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-244-01 1 in 1 CARTON 09/01/2012 1 30 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 09/01/2012 BB TINTED TREATMENT PRIMER BROAD SPECTRUM SPF 30 SUNSCREEN MEDIUM
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-245 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 45.5 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 35 mg in 1 mL Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) ISODODECANE (UNII: A8289P68Y2) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) MICA (UNII: V8A1AW0880) POLYMETHYLSILSESQUIOXANE (11 MICRONS) (UNII: Z570VEV8XK) HEXYL LAURATE (UNII: 4CG9F9W01Q) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) STEARIC ACID (UNII: 4ELV7Z65AP) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) ALUMINUM OXIDE (UNII: LMI26O6933) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) DIPALMITOYL HYDROXYPROLINE (UNII: E6AHA53N1H) DIAMOND (UNII: 6GRV67N0U2) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-245-01 1 in 1 CARTON 09/01/2012 1 30 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 09/01/2012 BB TINTED TREATMENT PRIMER BROAD SPECTRUM SPF 30 SUNSCREEN MEDIUM-TAN
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-246 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 45.5 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 35 mg in 1 mL Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) ISODODECANE (UNII: A8289P68Y2) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) MICA (UNII: V8A1AW0880) POLYMETHYLSILSESQUIOXANE (11 MICRONS) (UNII: Z570VEV8XK) HEXYL LAURATE (UNII: 4CG9F9W01Q) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) STEARIC ACID (UNII: 4ELV7Z65AP) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) ALUMINUM OXIDE (UNII: LMI26O6933) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) DIPALMITOYL HYDROXYPROLINE (UNII: E6AHA53N1H) DIAMOND (UNII: 6GRV67N0U2) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-246-01 1 in 1 CARTON 09/01/2012 1 30 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 09/01/2012 BB TINTED TREATMENT PRIMER BROAD SPECTRUM SPF 30 SUNSCREEN TAN
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-247 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 45.5 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 35 mg in 1 mL Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) ISODODECANE (UNII: A8289P68Y2) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) POLYMETHYLSILSESQUIOXANE (11 MICRONS) (UNII: Z570VEV8XK) HEXYL LAURATE (UNII: 4CG9F9W01Q) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) CYCLOMETHICONE 6 (UNII: XHK3U310BA) STEARIC ACID (UNII: 4ELV7Z65AP) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) ALUMINUM OXIDE (UNII: LMI26O6933) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) DIPALMITOYL HYDROXYPROLINE (UNII: E6AHA53N1H) DIAMOND (UNII: 6GRV67N0U2) MICA (UNII: V8A1AW0880) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-247-01 1 in 1 CARTON 09/01/2012 1 30 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 09/01/2012 BB TINTED TREATMENT PRIMER BROAD SPECTRUM SPF 30 SUNSCREEN TAN DEEP
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-248 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 45.5 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 35 mg in 1 mL Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) ISODODECANE (UNII: A8289P68Y2) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) POLYMETHYLSILSESQUIOXANE (11 MICRONS) (UNII: Z570VEV8XK) MICA (UNII: V8A1AW0880) HEXYL LAURATE (UNII: 4CG9F9W01Q) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) STEARIC ACID (UNII: 4ELV7Z65AP) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) ALUMINUM OXIDE (UNII: LMI26O6933) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) DIPALMITOYL HYDROXYPROLINE (UNII: E6AHA53N1H) DIAMOND (UNII: 6GRV67N0U2) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-248-01 1 in 1 CARTON 09/01/2012 1 30 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 09/01/2012 BB TINTED TREATMENT PRIMER BROAD SPECTRUM SPF 30 SUNSCREEN DEEP
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-249 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 45.5 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 35 mg in 1 mL Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) ISODODECANE (UNII: A8289P68Y2) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) POLYMETHYLSILSESQUIOXANE (11 MICRONS) (UNII: Z570VEV8XK) HEXYL LAURATE (UNII: 4CG9F9W01Q) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) DIAMOND (UNII: 6GRV67N0U2) STEARIC ACID (UNII: 4ELV7Z65AP) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) ALUMINUM OXIDE (UNII: LMI26O6933) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) DIPALMITOYL HYDROXYPROLINE (UNII: E6AHA53N1H) MICA (UNII: V8A1AW0880) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-249-01 1 in 1 CARTON 09/01/2012 1 30 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 09/01/2012 BB TINTED TREATMENT PRIMER BROAD SPECTRUM SPF 30 SUNSCREEN RICH
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-250 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 45.5 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 35 mg in 1 mL Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) ISODODECANE (UNII: A8289P68Y2) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) POLYMETHYLSILSESQUIOXANE (11 MICRONS) (UNII: Z570VEV8XK) HEXYL LAURATE (UNII: 4CG9F9W01Q) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) CYCLOMETHICONE 6 (UNII: XHK3U310BA) STEARIC ACID (UNII: 4ELV7Z65AP) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) ALUMINUM OXIDE (UNII: LMI26O6933) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) DIPALMITOYL HYDROXYPROLINE (UNII: E6AHA53N1H) DIAMOND (UNII: 6GRV67N0U2) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) MICA (UNII: V8A1AW0880) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-250-01 1 in 1 CARTON 09/01/2012 1 30 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 09/01/2012 BB TINTED TREATMENT PRIMER BROAD SPECTRUM SPF 30 SUNSCREEN MAHOGANY
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-251 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 45.5 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 35 mg in 1 mL Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) ISODODECANE (UNII: A8289P68Y2) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) POLYMETHYLSILSESQUIOXANE (11 MICRONS) (UNII: Z570VEV8XK) HEXYL LAURATE (UNII: 4CG9F9W01Q) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) MICA (UNII: V8A1AW0880) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) STEARIC ACID (UNII: 4ELV7Z65AP) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) ALUMINUM OXIDE (UNII: LMI26O6933) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) DIPALMITOYL HYDROXYPROLINE (UNII: E6AHA53N1H) DIAMOND (UNII: 6GRV67N0U2) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-251-01 1 in 1 CARTON 09/01/2012 1 30 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 09/01/2012 BB TINTED TREATMENT PRIMER BROAD SPECTRUM SPF 30 SUNSCREEN FAIR-LIGHT
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-364 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 45.5 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 35 mg in 1 mL Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) ISODODECANE (UNII: A8289P68Y2) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) HEXYL LAURATE (UNII: 4CG9F9W01Q) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) DIAMOND (UNII: 6GRV67N0U2) STEARIC ACID (UNII: 4ELV7Z65AP) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) ALUMINUM OXIDE (UNII: LMI26O6933) DIPALMITOYL HYDROXYPROLINE (UNII: E6AHA53N1H) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) MICA (UNII: V8A1AW0880) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-364-01 1 in 1 CARTON 09/01/2012 1 30 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 09/01/2012 BB TINTED TREATMENT PRIMER BROAD SPECTRUM SPF 30 SUNSCREEN LIGHT-MEDIUM
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-359 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 45.5 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 35 mg in 1 mL Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) ISODODECANE (UNII: A8289P68Y2) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) HEXYL LAURATE (UNII: 4CG9F9W01Q) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) DIAMOND (UNII: 6GRV67N0U2) STEARIC ACID (UNII: 4ELV7Z65AP) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) ALUMINUM OXIDE (UNII: LMI26O6933) DIPALMITOYL HYDROXYPROLINE (UNII: E6AHA53N1H) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) MICA (UNII: V8A1AW0880) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-359-01 1 in 1 CARTON 09/01/2012 1 30 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 09/01/2012 BB TINTED TREATMENT PRIMER BROAD SPECTRUM SPF 30 SUNSCREEN DEEP-SAND
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-360 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 45.5 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 35 mg in 1 mL Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) ISODODECANE (UNII: A8289P68Y2) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) HEXYL LAURATE (UNII: 4CG9F9W01Q) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) DIAMOND (UNII: 6GRV67N0U2) STEARIC ACID (UNII: 4ELV7Z65AP) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) ALUMINUM OXIDE (UNII: LMI26O6933) DIPALMITOYL HYDROXYPROLINE (UNII: E6AHA53N1H) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) MICA (UNII: V8A1AW0880) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-360-01 1 in 1 CARTON 09/01/2012 1 30 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 09/01/2012 BB TINTED TREATMENT PRIMER BROAD SPECTRUM SPF 30 SUNSCREEN RICH HONEY
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-361 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 45.5 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 35 mg in 1 mL Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) ISODODECANE (UNII: A8289P68Y2) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) HEXYL LAURATE (UNII: 4CG9F9W01Q) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) DIAMOND (UNII: 6GRV67N0U2) STEARIC ACID (UNII: 4ELV7Z65AP) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) ALUMINUM OXIDE (UNII: LMI26O6933) DIPALMITOYL HYDROXYPROLINE (UNII: E6AHA53N1H) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) MICA (UNII: V8A1AW0880) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-361-01 1 in 1 CARTON 09/01/2012 1 30 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 09/01/2012 BB TINTED TREATMENT PRIMER BROAD SPECTRUM SPF 30 SUNSCREEN MAHOGANY HONEY
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-362 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 45.5 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 35 mg in 1 mL Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) ISODODECANE (UNII: A8289P68Y2) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) HEXYL LAURATE (UNII: 4CG9F9W01Q) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) DIAMOND (UNII: 6GRV67N0U2) STEARIC ACID (UNII: 4ELV7Z65AP) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) ALUMINUM OXIDE (UNII: LMI26O6933) DIPALMITOYL HYDROXYPROLINE (UNII: E6AHA53N1H) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) MICA (UNII: V8A1AW0880) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-362-01 1 in 1 CARTON 09/01/2012 1 30 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 09/01/2012 BB TINTED TREATMENT PRIMER BROAD SPECTRUM SPF 30 SUNSCREEN ESPRESSO
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-363 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 45.5 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 35 mg in 1 mL Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) ISODODECANE (UNII: A8289P68Y2) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) HEXYL LAURATE (UNII: 4CG9F9W01Q) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) DIAMOND (UNII: 6GRV67N0U2) STEARIC ACID (UNII: 4ELV7Z65AP) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) ALUMINUM OXIDE (UNII: LMI26O6933) DIPALMITOYL HYDROXYPROLINE (UNII: E6AHA53N1H) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) MICA (UNII: V8A1AW0880) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-363-01 1 in 1 CARTON 09/01/2012 1 30 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 09/01/2012 Labeler - Tarte, Inc. (027905186)