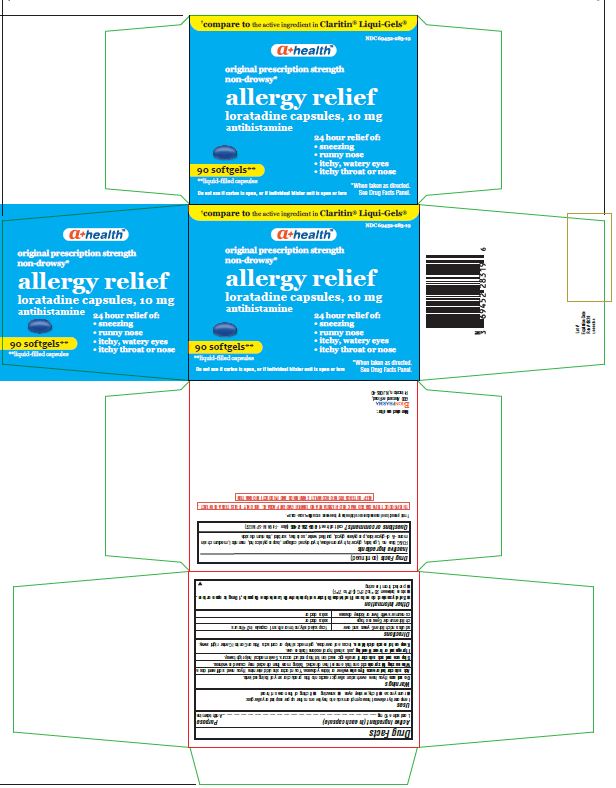
Label: LORATADINE capsule, liquid filled
- NDC Code(s): 69452-283-17, 69452-283-19, 69452-283-25
- Packager: Bionpharma Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 29, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each capsule)
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product
do not take more than directed. Taking more than directed may cause drowsiness.
- Directions
-
Other information
- Tamper-evident: do not use if foil seal under cap, printed with “Sealed for your protection” is missing, open or broken. (For Bottle Labels and Cartons)
- Safety sealed: do not use if individual blister unit printed with Loratadine Capsule, 10 mg is open or torn. (For Blister Carton)
- store between 20° to 25°C (68° to 77°F)
- protect from freezing
- Inactive ingredients
-
Questions or comments?
call toll free 1-888-235-2466 (Mon - Fri 9AM - 5PM EST)
†This product is not manufactured or distributed by the owners of Claritin® LIQUI-GELS®.
Blister carton contain the following:
THIS PRODUCT IS PACKAGED IN A CHILD-RESISTANT AND TAMPER EVIDENT PACKAGE. USE ONLY IF BLISTERS ARE INTACT.
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION.Do not use if carton is open, or if individual blister unit is open or torn
Manufactured for:
Bionpharma Inc.
600 Alexander Road,
Princeton, NJ 08540
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
LORATADINE
loratadine capsule, liquid filledProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69452-283 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LORATADINE (UNII: 7AJO3BO7QN) (LORATADINE - UNII:7AJO3BO7QN) LORATADINE 10 mg Inactive Ingredients Ingredient Name Strength CAPRYLIC/CAPRIC MONO/DI-GLYCERIDES (UNII: U72Q2I8C85) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) GELATIN (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) HYPROMELLOSES (UNII: 3NXW29V3WO) HYDROLYSED BOVINE COLLAGEN (ENZYMATIC; 2000-5000 MW) (UNII: 5WE8P977RQ) ISOPROPYL ALCOHOL (UNII: ND2M416302) MANNITOL (UNII: 3OWL53L36A) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SORBITAN (UNII: 6O92ICV9RU) SORBITOL (UNII: 506T60A25R) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color blue Score no score Shape OVAL Size 10mm Flavor Imprint Code L10 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69452-283-19 1 in 1 CARTON 03/20/2020 1 90 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:69452-283-25 180 in 1 BOTTLE; Type 0: Not a Combination Product 03/20/2020 3 NDC:69452-283-17 1 in 1 CARTON 08/26/2021 3 60 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA202538 03/20/2020 Labeler - Bionpharma Inc. (079637826) Registrant - Bionpharma Inc. (079637826) Establishment Name Address ID/FEI Business Operations Patheon Softgels Inc. 002193829 manufacture(69452-283)