Label: MOTION SICKNESS RELIEF- meclizine hcl tablet, chewable
- NDC Code(s): 51316-404-21
- Packager: CVS WOONSOCKET PRESCRIPTION CENTER, INCORPORATED
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 23, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each chewable tablet)
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
- glaucoma
- difficulty in urination due to enlargement of the prostate gland
- a breathing problem such as emphysema or chronic bronchitis
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
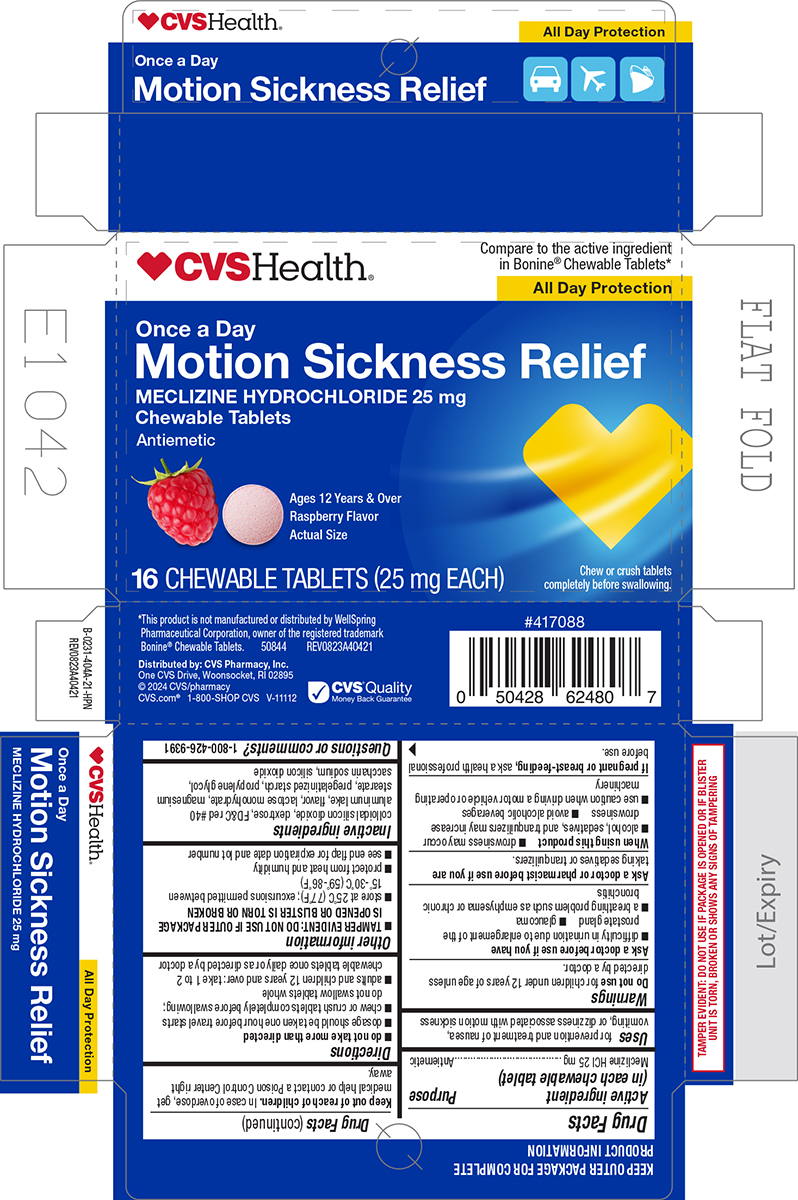
Principal Display Panel - CVS
♥CVSHealth®
Compare to the active ingredient
in Bonine® Chewable Tablets*
All Day ProtectionOnce a Day
Motion Sickness Relief
MECLIZINE HYDROCHLORIDE 25 mg
Chewable Tablets
AntiemeticAges 12 Years & Over
Raspberry Flavor
Actual Size16 CHEWABLE TABLETS (25 mg EACH)
Chew or crush tablets
completely before swallowing.*This product is not manufactured or distributed by WellSpring
Pharmaceutical Corporation, owner of the registered trademark
Bonine® Chewable Tablets. 50844 REV0823A40421Distributed by: CVS Pharmacy, Inc.
One CVS Drive, Woonsocket, RI 02895
© 2024 CVS/pharmacy
CVS.com® 1-800-SHOP CVS V-11112TAMPER EVIDENT: DO NOT USE IF PACKAGE IS OPENED OR IF BLISTER
UNIT S TORN, BROKEN OR SHOWS ANY SIGNS OF TAMPERING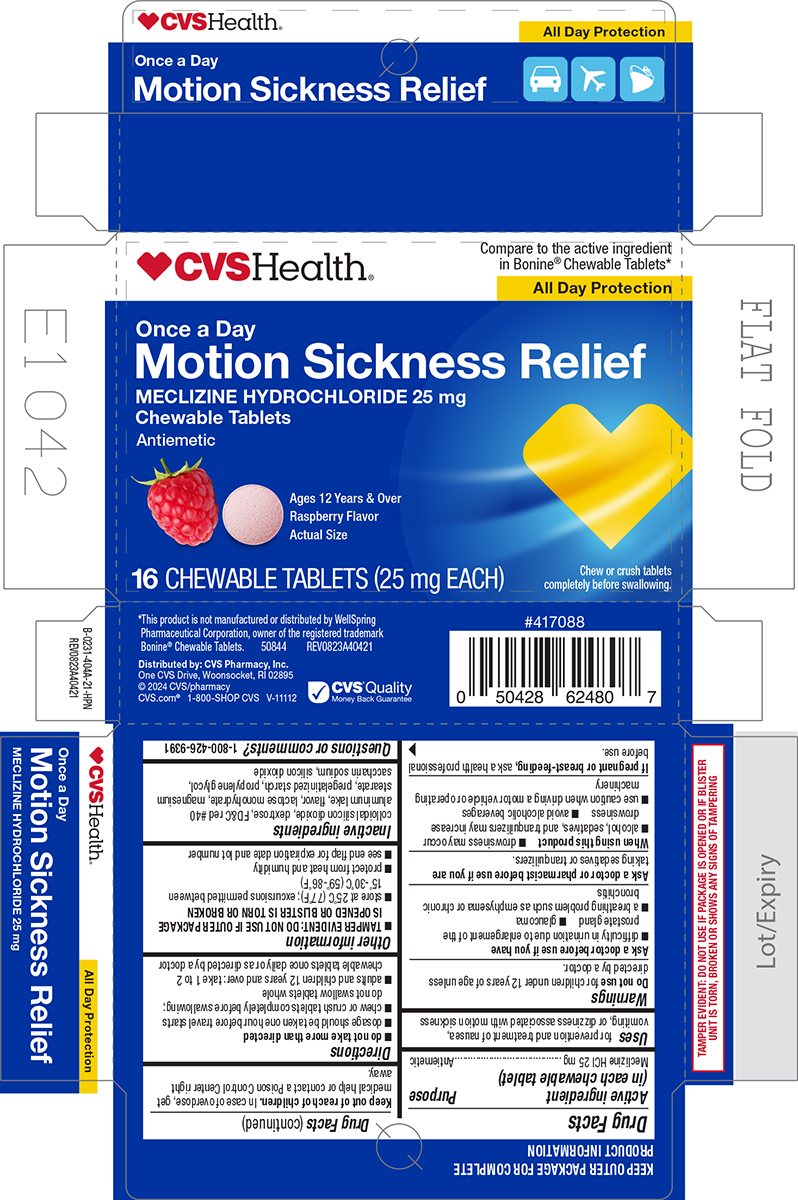
CVS 44-404A
-
INGREDIENTS AND APPEARANCE
MOTION SICKNESS RELIEF
meclizine hcl tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51316-404 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MECLIZINE HYDROCHLORIDE (UNII: HDP7W44CIO) (MECLIZINE - UNII:3L5TQ84570) MECLIZINE HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DEXTROSE, UNSPECIFIED FORM (UNII: IY9XDZ35W2) FD&C RED NO. 40 (UNII: WZB9127XOA) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) STARCH, CORN (UNII: O8232NY3SJ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SACCHARIN SODIUM (UNII: SB8ZUX40TY) Product Characteristics Color pink Score no score Shape ROUND Size 9mm Flavor RASPBERRY Imprint Code 44;404 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51316-404-21 2 in 1 CARTON 12/27/2023 1 8 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M009 12/27/2023 Labeler - CVS WOONSOCKET PRESCRIPTION CENTER, INCORPORATED (062312574) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867837 manufacture(51316-404) , pack(51316-404) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 117025878 manufacture(51316-404)