Label: CARE SCIENCE ESSENTIAL FIRST AID- ethyl alcohol, isopropyl alcohol, bacitracin zinc, neomycin sulfate, polymyxin-b sulfate kit
- NDC Code(s): 51142-001-01, 51142-445-21, 61010-2017-0, 61010-5600-1
- Packager: ASO LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated May 7, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
CONTENTS – Care Science Essential First Aid Kit
Adhesive Bandages
30 Sheer Bandages - 5/8 IN X 2 1/4 IN (15 MM X 57 MM)
20 Sheer Bandages - 3/4 IN X 3 IN (19 MM X 76 MM)
12 Butterfly Closures - 1 3/4 IN X 3/8 IN (44 MM X 9.5 MM)
Instruments
2 Wooden Finger Splints
2 Nitrile Gloves
1 First Aid Guide
1 Instant Cold Pack
1 Metal Tweezers
1 Paper Tape Roll - 1/2 IN X 5 YDS (12 MM X 4.5 M)
Topicals & Ointments
18 Alcohol Prep Pads - 1 3/16 IN X 2 5/16 IN (30 MM X 58 MM)
10 Triple Antibiotic Ointment - 1/32 OZ (0.9 G)
6 Antiseptic Wipes - 4 3/4 IN X 7 3/4 IN (120 MM X 196 MM)
Wound Dressings
4 Gauze Pads - 2 IN X 2 IN (50 MM X 50 MM)
2 Gauze Pads - 4 IN X 4 IN (101 MM x 101 MM)Made in USA with globally sourced materials: Antiseptic Wipes, Butterfly Closures, First Aid Guide, Triple Antibiotic
Ointment, Wooden Finger Splints.
Made in Philippines: Adhesive BandagesMade in China: Alcohol Prep Pads, Gauze Pads, Instant Cold Pack, Metal Tweezers, Nitrile Gloves, Paper Tape.
Made in Mexico:Carrying Case
Alcohol Prep Pads & Triple Antibiotic Ointment
Caution:This product contains non-prescription drug products that have expiration dates. Please check before use. Keep these and all drug products out of reach of children. Tamper evident sealed packets; do not use any opened or torn packets.
Adhesive Bandages:For use on minor cuts, scrapes, & burns.
Directions:For optimal results, apply bandage to clean, dry skin. Change the dressing daily, when wet, or more often if needed. Single use.Warning:For medical emergencies, seek professional help.
Butterfly Closures:For use on minor cuts, scrapes, & burns.
Directions:Remove backing tabs from Butterfly Closure. Apply adhesive sections on either side of the wound. Be sure to center the nonstick area over the wound. Change dressing as directed by your health care professional.Warning:For medical emergencies, seek professional help.
Gauze Pads:For cleaning wounds.
Directions:Gently clean the wound with mild soap and water using the gauze pad and carefully dry the affected area. Discard the used pad.Warning:In case of deep puncture wounds or serious burns, consult a physician.
Nitrile Exam Gloves:Non-sterile single use disposable exam gloves.
Storage: Protect from freezing. Avoid excessive heat. Keep dry. Gloves should be shielded from direct sunlight, fluorescent lighting, x-rays, moisture and Ozone.Disposal:Dispose of gloves and all biologically contaminated matter in an appropriate container.
Paper Tape:For securing wound covers.
Directions:Use gauze to gently clean in and around injured area with mild soap and water. Dry injured area and apply medication if necessary. Cover wound with non-stick pad or dressing. Secure the dressing with paper tape to help keep out dirt and contaminants.Warning:In case of deep puncture wounds or serious burns, consult a physician.
Kit contents are not made with natural rubber latex.Adhesive Bandages, Butterfly Closures, and Gauze Pads are sterile unless wrapper is opened or damaged.
Gauze pads are rayon-polyester blend.
Actual appearance of contents may vary depending on availability. We reserve the right to add, substitute, or replace items at our own discretion. At no time will the integrity of the items be less than that which is indicated on the packaging.
See inside of clear plastic case for complete drug facts & warnings for Alcohol Prep Pad, Triple Antibiotic Ointment and Antiseptic Wipes.
CARE SCIENCE ® is an ASO brand
Distributed by ASO LLC | Sarasota, Fl 34240
www.asocorp.com
- Active ingredient – Alcohol Prep Pads
- Purpose – Alcohol Prep Pads
- Uses – Alcohol Prep Pads
-
Warnings – Alcohol Prep Pads
For external use only
Flammable, keep away from fire or flameDo not use – Alcohol Prep Pads
- with electrocautery procedures
- In the eyes.
If contact occurs, flush eyes with water.
- Directions – Alcohol Prep Pads
- Other information – Alcohol Prep Pads
- Inactive Ingredients – Alcohol Prep Pads
- Questions or comments? – Alcohol Prep Pads
- Active ingredient – Antiseptic Wipes
- Purpose – Antiseptic Wipes
- Uses – Antiseptic Wipes
- Warnings – Antiseptic Wipes
- Directions – Antiseptic Wipes
- Inactive ingredients – Antiseptic Wipes
- Questions or comments? – Antiseptic Wipes
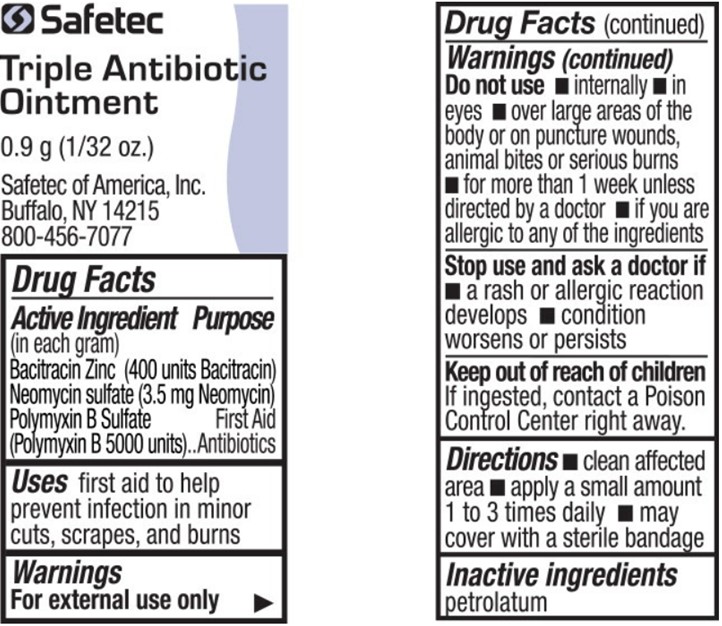
- Active ingredients (in each gram) – Triple Antibiotic Ointment
- Purpose – Triple Antibiotic Ointment
- Uses – Triple Antibiotic Ointment
-
Warnings – Triple Antibiotic Ointment
For external use only
Do not use – Triple Antibiotic Ointment
in the eyes. If this happens, rinse thoroughly with water.
- internally
- over large areas of the body
- if you are allergic to any of the ingredients
- longer than 1 week unless directed by a doctor
Ask a doctor before use if you have – Triple Antibiotic Ointment
- deep or puncture wounds
- animal bites
- serious burns
- Directions – Triple Antibiotic Ointment
- Other information – Triple Antibiotic Ointment
- Inactive ingredient – Triple Antibiotic Ointment
- Questions or comments? – Triple Antibiotic Ointment
- Principal Display Panel – Care Science Essential First Aid Kit
- Principal Display Panel – Alcohol Prep Pads
- Principal Display Panel – Antiseptic Wipes
- Principal Display Panel – Triple Antibiotic Ointment
-
INGREDIENTS AND APPEARANCE
CARE SCIENCE ESSENTIAL FIRST AID
ethyl alcohol, isopropyl alcohol, bacitracin zinc, neomycin sulfate, polymyxin-b sulfate kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51142-001 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51142-001-01 1 in 1 CASE; Type 0: Not a Combination Product 03/11/2021 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 12 POUCH 4.08 g Part 2 6 PACKET 0.0114 L Part 3 10 POUCH 9 g Part 1 of 3 ALCOHOL PREP PAD
alcohol prep pad swabProduct Information Item Code (Source) NDC:51142-445 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 70 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Product Characteristics Color white (White pad) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51142-445-21 0.34 g in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 09/24/2018 Part 2 of 3 ANTISEPTIC
alcohol clothProduct Information Item Code (Source) NDC:61010-2017 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 665 mL in 1 L Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) WATER (UNII: 059QF0KO0R) TROLAMINE (UNII: 9O3K93S3TK) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61010-2017-0 0.0019 L in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 05/01/2017 Part 3 of 3 TRIPLE ANTIBIOTIC
bacitracin zinc, neomycin sulfate, polymyxin b sulfate ointmentProduct Information Item Code (Source) NDC:61010-5600 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BACITRACIN ZINC (UNII: 89Y4M234ES) (BACITRACIN - UNII:58H6RWO52I) BACITRACIN 400 [USP'U] in 1 g NEOMYCIN SULFATE (UNII: 057Y626693) (NEOMYCIN - UNII:I16QD7X297) NEOMYCIN 3.5 mg in 1 g POLYMYXIN B SULFATE (UNII: 19371312D4) (POLYMYXIN B - UNII:J2VZ07J96K) POLYMYXIN B 5000 [USP'U] in 1 g Inactive Ingredients Ingredient Name Strength PETROLATUM (UNII: 4T6H12BN9U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61010-5600-1 0.9 g in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M004 08/08/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 03/11/2021 Labeler - ASO LLC (152793493)