Label: COCAINE HYDROCHLORIDE NASAL solution
- NDC Code(s): 81665-301-02
- Packager: OMNIVIUM PHARMACEUTICALS LLC.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CII
- Marketing Status: New Drug Application Authorized Generic
Drug Label Information
Updated September 24, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use COCAINE HYDROCHLORIDE safely and effectively. See full prescribing information for COCAINE HYDROCHLORIDE.
Cocaine Hydrochloride nasal solution, CII
Initial U.S. Approval: 2017WARNING: ABUSE AND DEPENDENCE
See full prescribing information for complete boxed warning.
CNS stimulants, including cocaine hydrochloride, have a high potential for abuse and dependence. (5.1)
INDICATIONS AND USAGE
Cocaine hydrochloride nasal solution is an ester local anesthetic indicated for the induction of local anesthesia of the mucous membranes when performing diagnostic procedures and surgeries on or through the nasal cavities in adults. (1)
DOSAGE AND ADMINISTRATION
- For intranasal use only. (2.1)
- Do not apply to damaged nasal mucosa. (2.1)
- Cocaine hydrochloride should not be substituted for other nasal cocaine products unless determined by FDA to be substitutable. (2.1)
- The recommended dose of cocaine hydrochloride ranges from 40 mg to 160 mg, depending on the nasal surface area to be anesthetized and the procedure to be performed. (2.2)
Cocaine hydrochloride should be administered by means of cotton or rayon applicator pledgets applied to the nasal mucosa. (2)
- One pledget will absorb one mL of cocaine hydrochloride nasal solution. (2.2)
- Preparation and Application:
- Draw up 4 mL cocaine hydrochloride 4% nasal solution into a syringe calibrated in mL. Apply 2 mL cocaine hydrochloride nasal solution to the top of four stacked pledgets. Turn the stacked pledgets over and apply 2 mL cocaine hydrochloride nasal solution to the other side. (2.3)
- Cocaine hydrochloride nasal solution should be evenly distributed on all pledgets. (2.3)
- Following soaking, place a maximum of two pledgets in each nasal cavity. (2.3)
Leave pledgets in place for up to 20 minutes. (2.3)
Pledgets should be removed immediately upon any sign or symptom of an adverse event. (2.2)
DOSAGE FORMS AND STRENGTHS
Nasal solution:
- 4% nasal solution (40 mg/ mL; available in both 4mL and 10mL bottles) (3)
CONTRAINDICATIONS
- Hypersensitivity to cocaine, or any component of cocaine hydrochloride. (4)
WARNINGS AND PRECAUTIONS
- Cocaine hydrochloride is for TOPICAL USE ONLY. NOT FOR INJECTION OR OPHTHALMIC USE. (5)
- Seizures: Cocaine hydrochloride may lower the convulsive threshold. Monitor patients for development of seizures.
- Blood Pressure and Heart Rate Increases: Monitor vital signs, including heart rate and rhythm, in patients after receiving cocaine hydrochloride. Avoid use of cocaine hydrochloride in patients with a recent or active history of myocardial infarction, coronary artery disease, congestive heart failure, irregular heart rhythm, abnormal ECG, uncontrolled hypertension, or thyrotoxicosis.
ADVERSE REACTIONS
The most common adverse reactions (> 1%) occurring in patients treated with cocaine hydrochloride were hypertension, tachycardia, and sinus tachycardia. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Omnivium Pharmaceuticals LLC at 1-888-807-1048 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Epinephrine, Phenylephrine: There have been reports of myocardial ischemia, myocardial infarction, and ventricular arrhythmias with concomitant use during nasal surgery. Avoid use of additional vasoconstrictor agents with cocaine hydrochloride. If concomitant use is unavoidable, prolonged vital sign and ECG monitoring may be required. (5.3, 7.2)
- Disulfiram: Disulfiram treatment increases plasma cocaine exposure. Avoid using cocaine hydrochloride in patients taking disulfiram. (7.3)
- CNS stimulants: Concomitant administration may result in nervousness, irritability, or possibly convulsions. (7.1)
- Cholinesterase inhibitors: Concomitant administration may increase the risk of cocaine toxicity. (7.4)
- Sympathomimetics, postganglionic blocking agents, and tricyclic antidepressants: Concomitant administration may increase the risk of cardiovascular adverse reactions. (7.5, 7.6)
- Monoamine-oxidase inhibitors: Concomitant administration may potentiate the effects and toxicity of monoamine-oxidase inhibitors. (7.7)
USE IN SPECIFIC POPULATIONS
- Lactation: Breastfeeding not recommended during treatment, but a lactating woman can pump and discard breast milk for 48 hours after treatment. (8.2)
- Geriatric Patients: May be more susceptible to cardiovascular adverse events. (8.5)
- Hepatic impairment: It is not recommended to dose cocaine hydrochloride in patients with hepatic impairment. (8.6)
- Renal impairment: Monitor for adverse reactions such as headache, epistaxis, and clinically-relevant increases in heart rate or blood pressure. (8.7)
- Pseudocholinesterase deficiency. (8.8)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 8/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage and Administration Instructions
2.2 Dosing Recommendations
2.3 Preparation and Administration of Cocaine Hydrochloride via Pledgets
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Potential for Abuse and Dependence
5.2 Seizures
5.3 Blood Pressure and Heart Rate Increases
5.4 Toxicology Screening
5.5 Known Hypersensitivity or Idiosyncrasy to the Sympathomimetic Amines
5.6 Ophthalmic Use
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Central Nervous System Stimulants
7.2 Epinephrine, Phenylephrine
7.3 Disulfiram
7.4 Cholinesterase Inhibitors
7.5 Postganglionic Blocking Agents
7.6 Tricyclic Antidepressants
7.7 Monoamine-Oxidase Inhibitors
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
8.7 Renal Impairment
8.8 Pseudocholinesterase Deficiency
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
9.2 Abuse
9.3 Dependence
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: ABUSE AND DEPENDENCE
CNS stimulants, including cocaine hydrochloride, have a high potential for abuse and dependence. (5.1)
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage and Administration Instructions
- Cocaine hydrochloride is for intranasal use only.
- Do not apply cocaine hydrochloride to damaged nasal mucosa.
- Unless the FDA has determined that these products can be substituted, do not substitute cocaine hydrochloride for other intranasal cocaine products because this may result in different local and/or systemic exposures.
2.2 Dosing Recommendations
The recommended dose of cocaine hydrochloride ranges from 40 mg to 160 mg, depending on the nasal mucosal area to be anesthetized and the procedure to be performed. Each pledget absorbs one mL of cocaine hydrochloride nasal solution. A maximum of two soaked cotton or rayon pledgets may be placed in each nasal cavity, for a total dose of 160 mg for cocaine hydrochloride nasal solution 4%.
The total dose for any one procedure or surgery should not exceed 3 mg/kg cocaine hydrochloride.
The recommended size of the cotton or rayon pledgets for use with cocaine hydrochloride measure ½ inch x 3 inch (sold separately).
2.3 Preparation and Administration of Cocaine Hydrochloride via Pledgets
Draw up 4 mL cocaine hydrochloride into a syringe calibrated in mL. Stack four pledgets and apply 2 mL of solution to the top of the stacked pledgets. Turn the stacked pledgets over and apply 2 mL of solution to the other side. Cocaine hydrochloride should be evenly distributed on all pledgets.
Following cocaine hydrochloride application to pledgets, place One (1) or two (2) pledgets in each nasal cavity, for a maximum of 2 pledgets used per nostril.
Leave pledgets in place for up to 20 minutes. Remove pledgets and continue with the procedure. Discard pledgets, and dispose of any unused pledgets and remaining solution in accordance with institutional procedures for CII products.
Pledgets should be removed immediately upon any sign or symptom of an adverse event.
-
3 DOSAGE FORMS AND STRENGTHS
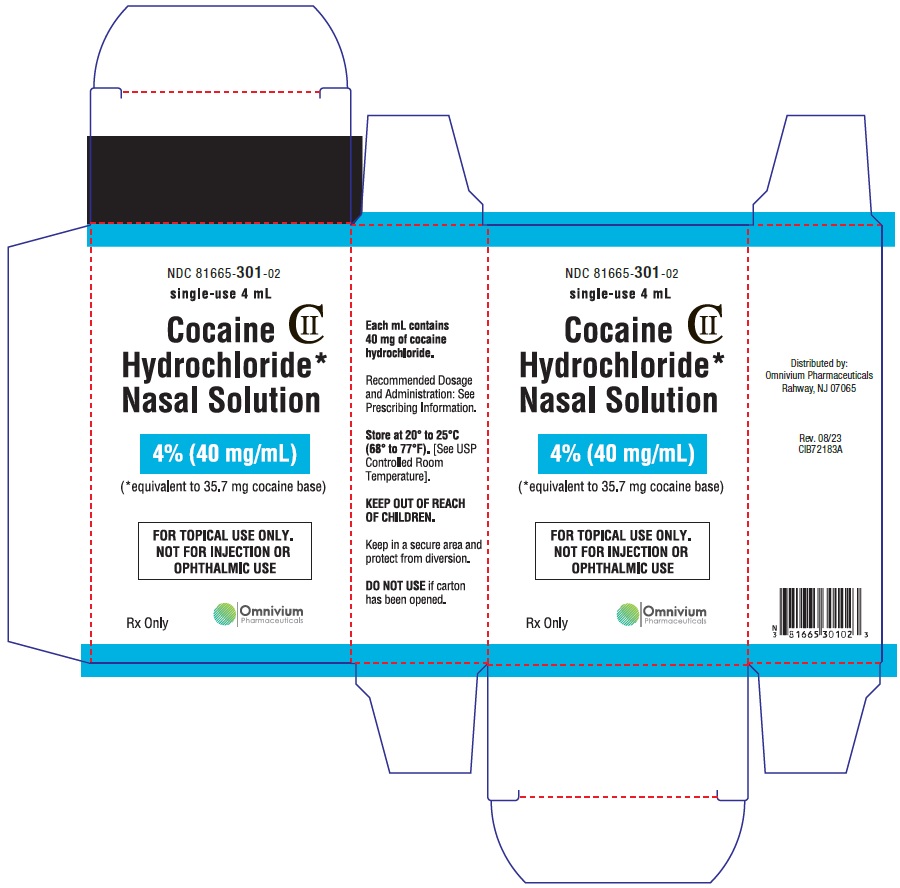
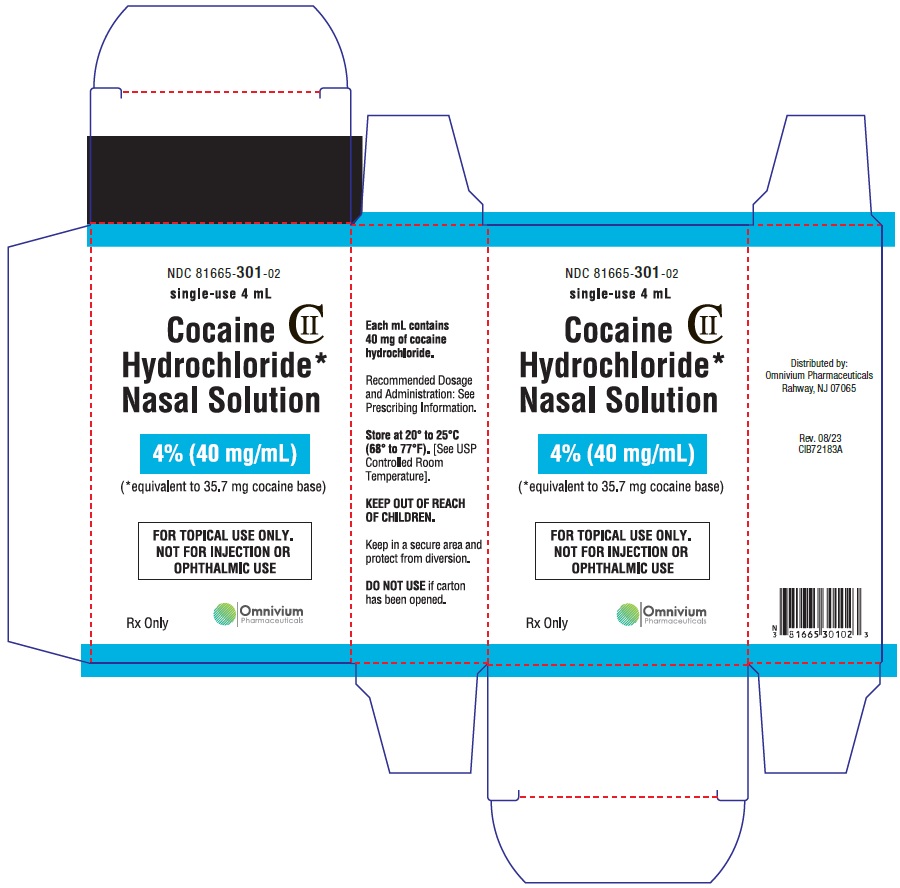
Cocaine Hydrochloride nasal solution is a clear, blue-green solution provided in a concentration of 4% (40 mg/mL). The 4% nasal solution is provided in both a single-use 4 mL (160 mg/4 mL) and multiple-use 10 mL (400 mg/10 mL) bottles. Each 1 mL of the 4% solution contains 40 mg of cocaine hydrochloride, equivalent to 35.7 mg of cocaine free base as aqueous solution, for topical nasal administration.
-
4 CONTRAINDICATIONS
Cocaine hydrochloride is contraindicated in:
- patients with a known history of hypersensitivity to cocaine hydrochloride, other ester-based local anesthetics, or any other component of the nasal solution [see Warnings and Precautions (5.5)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Potential for Abuse and Dependence
Central nervous system (CNS) stimulants, including cocaine hydrochloride, have a high potential for abuse and dependence [see Drug Abuse and Dependence (9.2, 9.3)].
5.2 Seizures
It has been reported in the literature that cocaine hydrochloride may lower the convulsive threshold. The risk may be higher in patients with a history of seizures or in patients with prior electroencephalogram (EEG) abnormalities without seizures, but has been reported in patients with no prior history or EEG evidence of seizures. Monitor patients for development of seizures.
5.3 Blood Pressure and Heart Rate Increases
As reported in the literature, cocaine hydrochloride causes an increase in observed blood pressure and heart rate. In the Phase 3 clinical studies with cocaine hydrochloride, increases in blood pressure and heart rate were observed following pledget removal. Monitor for changes in vital signs, including heart rate and rhythm, after administration of cocaine hydrochloride.
Avoid use of cocaine hydrochloride in patients with a history of myocardial infarction, coronary artery disease, congestive heart failure, irregular heart rhythm, abnormal ECG, or uncontrolled hypertension. Avoid use of additional vasoconstrictor agents such as epinephrine or phenylephrine with cocaine hydrochloride. If concomitant use is unavoidable, prolonged vital sign and ECG monitoring may be required.
Although not reported in the cocaine hydrochloride clinical trials, myocardial infarction has been reported in the literature, and can occur when the drug has been used as recommended [see Adverse Reactions (6)].
5.4 Toxicology Screening
The time after cocaine administration for which cocaine and its metabolites can be detected in plasma and urine depends on the sensitivity of the utilized assay method. The cocaine hydrochloride and its metabolites in cocaine hydrochloride may be detected in plasma for up to one week after administration. Cocaine hydrochloride and its metabolites may be detected in urine toxicology screening for longer than one week after administration.
5.5 Known Hypersensitivity or Idiosyncrasy to the Sympathomimetic Amines
Cocaine hydrochloride is contraindicated in patients with a known history of hypersensitivity to cocaine or to the components of the nasal solution. Cocaine is a sympathetic neuronal catecholamine reuptake inhibitor, which may potentiate the actions of concomitantly administered sympathomimetic amines.
-
6 ADVERSE REACTIONS
The following treatment-emergent adverse events are discussed in more detailed in other sections of the labeling:
- Increases in Blood Pressure and Heart Rate [see Warnings and Precautions (5.3)]
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Cocaine hydrochloride nasal solution has been evaluated in one Phase 1 study, one QT study and two Phase 3 studies, which included 702 adult subjects who received a single application of cocaine hydrochloride nasal solution 4%, cocaine hydrochloride nasal solution 10%, or placebo. The randomized, double-blind, placebo controlled Phase 3 studies were conducted in adult patients undergoing diagnostic procedures and surgeries on or through the mucous membranes of the nasal cavities, of which 316 received cocaine hydrochloride nasal solution 4%, 318 received cocaine hydrochloride nasal solution 10%, and 168 received placebo. Safety was evaluated for up to 7 days after dosing.
In a Phase 3 study, patients received a mean dose of 126 mg (80 to 160 mg, N=259) of cocaine hydrochloride using cocaine hydrochloride nasal solution 4% and a mean dose of 319 mg (200 to 400 mg, N=259) of cocaine hydrochloride using cocaine hydrochloride nasal solution 10% as a single application.
The most common adverse reactions reported with cocaine hydrochloride 4% are included in Table 1 (preexisting nasal conditions are not included). There were two patients treated with cocaine hydrochloride nasal solution 4% who withdrew due to an adverse reaction. One patient developed anxiety and systolic hypertension and one patient developed intermittent paroxysmal tachycardia. Both patients developed symptoms within 10 minutes of nasal pledget application. Three patients treated with cocaine hydrochloride nasal solution 10% required premature removal of pledgets due to nausea and diastolic hypertension; mild intermittent paroxysmal hypertension and paroxysmal tachycardia; and vasovagal syncope with bradycardia.
Table 1. Common Adverse Reactions with Cocaine Hydrochloride in > 1% of Treated Patients
MedDRA System Organ Class and Preferred Term Cocaine Hydrochloride, 4%
(N=259) n, %
Cocaine Hydrochloride, 10%
(N=259) n, %
Placebo
(N=128) n, %
Vascular Disorders 203 (78) 224 (87) 86 (67) Hypertension 201 (78) 220 (85) 85 (66) Cardiac Disorders 31 (12) 47 (18) 10 (8) Tachycardia 12 (5) 28 (11) 1 (1) Bradycardia 8 (3) 1 (0.4) 5 (4) Sinus tachycardia 6 (2) 9 (4) 0 Investigations 13 (5) 30 (12) 8 (6) QRS prolonged 4 (2) 8 (3) 3 (2) QT interval prolonged 7 (3) 10 (4) 3 (2) 6.2 Postmarketing Experience
The following adverse reactions have been identified during use of Cocaine Hydrochloride Nasal Solution. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Nervous system disorders: Headache, Seizure
Cardiac disorders: Hypertension, tachycardia, atrial and ventricular arrhythmias, myocardial ischemia and infarction
Psychiatric disorders: Anxiety
-
7 DRUG INTERACTIONS
7.1 Central Nervous System Stimulants
Concurrent use of other central nervous system stimulants with cocaine may result in excessive stimulation, leading to nervousness, irritability, or possibly convulsions, or cardiac arrhythmias.
7.2 Epinephrine, Phenylephrine
There are reports in the published literature of myocardial ischemia, myocardial infarction, and ventricular arrhythmias after concomitant administration of topical intranasal cocaine with epinephrine and phenylephrine during nasal and sinus surgery.
Avoid use of additional vasoconstrictor agents such as epinephrine and phenylephrine with cocaine hydrochloride during nasal and sinus surgery. If concomitant use is unavoidable, prolonged vital sign and ECG monitoring may be required.
7.3 Disulfiram
Published literature reported that disulfiram treatment increased plasma cocaine exposure (AUC and Cmax), by several folds after acute intranasal cocaine administration. Another literature reported that co-administration of disulfiram increased AUC of plasma cocaine by several folds after intravenous cocaine administration [see Clinical Pharmacology (12.3)].
Avoid using cocaine hydrochloride in patients taking disulfiram. Consider using other local anesthesia.
7.4 Cholinesterase Inhibitors
Cocaine has been described in literature to be primarily metabolized and inactivated by non-enzymatic ester hydrolysis and hepatic carboxylesterase, and also by plasma cholinesterase, hepatic carboxylesterase, and CYP3A4. The pharmacokinetics of cocaine hydrochloride in patients with reduced plasma cholinesterase activity has not been studied.
Plasma cholinesterase activity may be decreased by chronic administration of certain monoamine oxidase inhibitors, oral contraceptives, glucocorticoids, antimyasthenics (neostigmine), cyclophosphamide, and possibly thiotepa.
It may also be diminished by administration of irreversible plasma cholinesterase inhibitors such as echothiophate, organophosphate insecticides, and certain antineoplastic agents. Patients with reduced plasma cholinesterase (pseudocholinesterase) activity may have reduced clearance and increased exposure of plasma cocaine after administration of cocaine hydrochloride.
Because cocaine is metabolized by multiple enzymes, the effect of reduced plasma cholinesterase activity on cocaine exposure may be limited. No dosage adjustment of cocaine hydrochloride is needed in patients with reduced plasma cholinesterase. Monitor patients with reduced plasma cholinesterase activity for adverse reactions such as clinically-relevant increases in heart rate or blood pressure.
7.5 Postganglionic Blocking Agents
Agents such as reserpine potentiate cocaine-induced sympathetic stimulation; concurrent use may increase the risk of hypertension and cardiac arrhythmias that may be life-threatening.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on the use of cocaine hydrochloride in pregnant women to identify a drug-associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes. Adverse maternal and fetal/neonatal outcomes have been seen in women with chronic cocaine abuse during pregnancy (see Data).
In published animal reproduction studies, cocaine administered to pregnant females during the gestational period produced hydronephrosis (0.5 times the human reference dose (HRD) of 37.5 mg via the 4% solution), developmental delays in the offspring (1.5 times the HRD), cerebral hemorrhage and fetal edema (2.0 times the HRD), reduced fetal body weights and brain weights (2.6 times the HRD), and reduced fetal survival (3.7 times the HRD).
Single dose administration of cocaine intravenously during organogenesis in mice produced cryptochidism, anophthalmia, exencephaly, and delayed ossification at 7.8 times the HRD. In rats, a single dose of cocaine administered by intraperitoneal injection produced edematous fetuses, hemorrhages and limb defects at 12.9 times the HRD (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population(s) are unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data
Human Data
There are no available data on the use of intranasal cocaine hydrochloride solution in pregnant women to inform a drug-associated risk for major congenital malformations or miscarriage. There are published data describing adverse developmental outcomes in women with chronic cocaine abuse during pregnancy. The published case-control and observational studies examining the effect of in utero cocaine exposure on fetal growth parameters, after controlling for confounding variables, found exposure was associated with reduced fetal growth compared with non-drug-abuse populations.
Published data from a large number of studies of women with chronic cocaine abuse during pregnancy are inconsistent in their findings with regard to congenital malformations, prematurity, miscarriage, premature rupture of membranes and gestational hypertension. The applicability of the findings from these studies of chronic abuse in pregnancy to a single topical exposure is limited.
Animal Data
Formal animal reproduction and development studies have not been conducted with intranasal cocaine hydrochloride. However, reproduction and development studies with cocaine have been reported in the published literature. Exposure margins below are based on body surface area comparison to the human reference dose (HRD) of 37.5 mg (estimated amount absorbed from the 160 mg (4%) cocaine-soaked pledgets).
Hydronephrosis was noted in embryos from pregnant rats treated wtih cocaine 2.1 mg/kg (0.5 times the HRD) and higher from Gestation Day 0 to 9. Cerebral hemorrhage and endematous fetuses were noted at 2.2 times the HRD and above).
Developmental delays and altered spontaneous exploratory behavior in response to cocaine were reported in rat pups born to dams treated intravenously with 6 mg/kg cocaine (1.5 times the HRD) from Gestation Day 8 to 20 in the absence of maternal toxicity.
Reduced fetal body and brain weights and alterations in fetal central neurotransmitter levels were noted following treatment of pregnant mice with 20 mg/kg cocaine from gestation days 8 to 12 or 12 to 18 (2.6 times the HRD).
Reduced fetal survival was noted when pregnant nonhuman primates were dosed with 0.3 mg/kg/h cocaine (3.7 times the HRD on per day basis) via a subcutaneous minipump from Gestation Day 24 to birth.
Exencephaly, cryptochidism, hydronephrosis, anophthalmia, delayed ossification, limb anomalies, and cerebral and intra-abdominal hemorrhage were reported following a single subcutaneous injection of 60 mg/kg cocaine (7.8 times the HRD) to pregant mice between Gestation Day 7 to 12. No significant maternal toxicity was reported at this dose.
Deficits in associational learning were reported when pregnant rats were treated with cocaine during gestation (10.3 times the HRD) in the absence of maternal toxicity.
Hemorrhage, fetal edema, and limb defects were reported when pregnant rats were administered a single injection of cocaine at a dose of 50 mg/kg/day or higher (12.9 times the HRD) during Gestation Day 9 to 19. Increased resorptions were noted at doses higher than 70 mg/kg/day (18.1 times the HRD) when administered on Gestation Day 16. No adverse effects were reported at a dose of 40 mg/kg (10.3 times the HRD).
Fetal deaths, decreased fetal body weights, edematous fetuses and single incidences of cleft palate and hypertrophic ventricle were observed when pregnant rats were treated with intraperitoneal cocaine at 60 mg/kg (15.5 times the HRD) from Gestation Day 8 to 12. Maternal toxicity was noted at this dose (mortality). No adverse effect level for fetal and maternal toxicity was noted at 50 mg/kg/day (13 times the HRD).
Decreased body weights, overall body length and crown circumference of offspring were reported when pregnant Rhesus monkeys were treated with escalating doses up to 7.5 mg/kg cocaine TID intramuscularly per day for 5 days per week from prior to conception to term (11.6 times the HRD).
8.2 Lactation
Risk Summary
Based on case reports in published literature, cocaine is present in human milk at widely varying concentrations. Based on its pharmacochemical characteristics, high concentrations of cocaine are expected in breast milk with systemic exposure. The applicability of these findings to a single topical exposure with limited systemic absorption is unclear. No studies have evaluated cocaine concentrations in milk after topical administration of cocaine hydrochloride.
Cocaine is detected in human breastmilk in chronic abuse situations and is expected to be at higher concentrations in milk than in maternal blood based on its physicochemical characteristics. Breastfeeding immediately after administration of cocaine hydrochloride could result in infant plasma concentrations that are approximately half the anticipated maximum maternal plasma concentrations at the clinical dose of 160 mg. The effects of this cocaine plasma concentration in an infant are unknown, but no level of cocaine exposure is considered safe for a breastfed infant.
Adverse reactions have occurred in infants ingesting cocaine through breastmilk, including vomiting, diarrhea, convulsions, hypertension, tachycardia, agitation and irritability. The long-term effects on infants exposed to cocaine through breast milk are unknown. There are no data on the effects of cocaine hydrochloride on milk production.
Because of the potential for serious adverse reactions in breastfed infants, advise a lactating woman that breastfeeding is not recommended during treatment with cocaine hydrochloride and to pump and discard breastmilk for 48 hours after use of cocaine hydrochloride.
8.3 Females and Males of Reproductive Potential
Published animal studies suggest that cocaine can alter female reproductive hormone levels, disrupt the estrous cycle, and reduce ovulation at doses approximately 1 to 2 times the HRD based on body surface area [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
Safety and effectiveness of cocaine hydrochloride in pediatric patients under the age of 18 have not been established.
Published studies state that in juvenile male rats, 30 mg/kg subcutaneous cocaine administration for longer than 7 days (7.8 times the HRD) produced testicular necrosis. Treatment of juvenile male rats with 15 mg/kg (3.9 times the HRD) for 100 days resulted in abnormal sperm morphology and reduced pregnancy rates.
8.5 Geriatric Use
Of the 802 subjects in the two Phase 3 studies with cocaine hydrochloride 13 subjects (1.6%) were age 65 and older, and one subject (0.1%) was 75 years of age or older.
No untoward or unexpected adverse reactions were seen in elderly patients who received cocaine hydrochloride compared to those subjects that were under the age of 65.
However, hypertension was observed in all geriatric subjects receiving cocaine hydrochloride. Special precaution should be given when determining the dose of cocaine hydrochloride for geriatric patients, commensurate with their age and physical status.
8.6 Hepatic Impairment
According to literature, cocaine is eliminated predominantly by metabolism in humans. The clearance of cocaine hydrochloride 4% has not been evaluated in subjects with hepatic impairment when compared to patients with normal hepatic function, and sufficient data are not available in literature to guide dosing in these subjects. Thus, cocaine hydrochloride should be avoided in patients with hepatic impairment [see Clinical Pharmacology (12.3)].
8.7 Renal Impairment
According to literature, cocaine is eliminated predominantly by metabolism in humans, with little excreted unchanged in the urine. The pharmacokinetics of cocaine hydrochloride in patients with renal impairment has not been studied. Based on information available on the metabolism and excretion of cocaine, dose initiation in patients with renal impairment should follow a conservative approach. Monitor patients with renal impairment for adverse reactions such as clinically-relevant increases in heart rate or blood pressure [see Clinical Pharmacology (12.3)].
8.8 Pseudocholinesterase Deficiency
Pharmacokinetics of cocaine hydrochloride in patients with reduced plasma cholinesterase activity has not been studied.
Genetic abnormalities of plasma cholinesterase (e.g., patients who are heterozygous or homozygous for atypical plasma cholinesterase gene), disease conditions such as malignant tumors, severe liver or kidney disease, decompensated heart disease, infections, burns, anemia, peptic ulcer, or myxedema or other physiological states such as pregnancy may lead to reduced plasma cholinesterase activity. Patients with reduced plasma cholinesterase (pseudocholinesterase) activity may have reduced clearance and increased exposure of plasma cocaine after administration of cocaine hydrochloride.
Because cocaine is metabolized by multiple enzymes, the effect of reduced plasma cholinesterase activity on cocaine exposure may be limited. No dosage adjustment of cocaine hydrochloride is needed in patients with reduced plasma cholinesterase. Monitor patients with reduced plasma cholinesterase activity for adverse reactions such as, clinically-relevant increases in heart rate or blood pressure [see Contraindications (4)].
-
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
Cocaine hydrochloride contains cocaine, a Schedule II controlled substance.
9.2 Abuse
Cocaine hydrochloride contains cocaine, a substance with a high potential for abuse. Cocaine hydrochloride can be misused and abused, which can lead to addiction. Cocaine hydrochloride may also be diverted for abuse purposes [see Warnings and Precautions (5.1)].
Drug abuse is the intentional non-therapeutic use of a prescription drug, even once, for its rewarding psychological or physiological effects. Drug addiction is a cluster of behavioral, cognitive, and physiological phenomena that develop after repeated substance use and includes: a strong desire to take the drug, difficulties in controlling its use, persisting in its use despite harmful consequences, a higher priority given to drug use than to other activities and obligations, increased tolerance, and sometimes a physical withdrawal. Drug abuse of a substance may occur without progression to drug addiction. “Drug-seeking” behavior is very common in persons with substance use disorders.
Drug abuse and addiction are conditions that are separate and distinct from physical dependence and tolerance [see Dependence (9.3)]. Health care providers should be aware that abuse and addiction may occur in the absence of symptoms indicative of physical dependence and tolerance.
Individuals who abuse stimulants may use cocaine hydrochloride for abuse purposes. Adverse events associated with abuse of cocaine include euphoria, excitation, irritability, restlessness, anxiety, paranoia, confusion, headache, psychosis, hypertension, stroke, seizures, dilated pupils, nausea, vomiting, and abdominal pain. Intranasal abuse can produce damage to the nostrils (e.g., ulceration and deviated septum). Abuse of cocaine can result in overdose, convulsions, unconsciousness, coma, and death [see Overdosage (10)].
Cocaine hydrochloride, like all prescription drugs with abuse potential, can be diverted for non-medical use into illicit channels of distribution. In order to minimize these risks, effective accounting procedures should be implemented, in addition to routine procedures for handling controlled substances.
9.3 Dependence
Physical dependence is a state that develops as a result of physiological adaptation in response to repeated drug use, manifested by withdrawal signs and symptoms after abrupt discontinuation or a significant dose reduction of a drug. Cocaine hydrochloride is approved for nasal single use, so physical dependence and withdrawal symptoms are unlikely to develop. Although cocaine hydrochloride is not indicated for chronic therapy, repeated misuse or abuse of this product may lead to physical dependence.
-
10 OVERDOSAGE
No cases of overdose with cocaine hydrochloride were reported in clinical trials. Reported blood pressure and heart rate increases were greater with cocaine hydrochloride nasal solution 10% than with cocaine hydrochloride nasal solution 4%.
In the case of an overdose, consult with a Certified Poison Control Center (1-800-222-1222) for up-to-date guidance and advice for treatment of overdosage. Individual patient response to cocaine varies widely. Toxic symptoms may occur idiosyncratically at low doses.
Manifestations of cocaine overdose associated with illicit use of cocaine reported in literature and based on reports in FDA’s Adverse Events Reporting System (AERS) database include death, cardio-respiratory arrest, cardiac arrest, respiratory arrest, tachycardia, myocardial infarction, agitation, aggression, restlessness, tremor, hyperreflexia, rapid respiration, confusion, assaultiveness, hallucinations, panic states, hyperpyrexia, and rhabdomyolysis. Fatigue and depression usually follow the central nervous system stimulation. Other reactions include arrhythmias, hypertension or hypotension, circulatory collapse, nausea, vomiting, diarrhea, and abdominal cramps. Fatal poisoning is usually preceded by convulsions and coma.
Because cocaine is significantly distributed to tissues and rapidly metabolized, dialysis and hemoperfusion are not effective. Acidification of the urine does not significantly enhance cocaine elimination.
-
11 DESCRIPTION
Cocaine Hydrochloride nasal solution is a clear, blue-green, aqueous solution, available in 4% strength. Each 1 mL contains cocaine hydrochloride 40 mg, equivalent to 35.7 mg of cocaine free base; 4% as 160 mg/4 mL or 400 mg/10 mL.
Cocaine, (1R,2R,3S,5S) methyl 3-(benzoyloxy)-8-methyl-8-azabicyclo[3.2.1]octane-2-carboxylate hydrochloride, is a synthetic tropane alkaloid ester, local anesthetic, which occurs as colorless to white crystals or white crystalline powder.
The structural formula for cocaine hydrochloride is as follows:
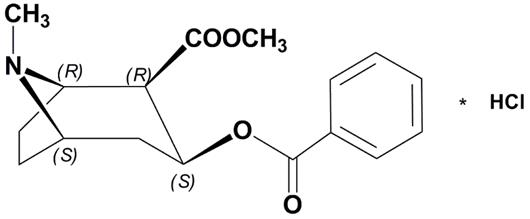
Formula C17H21NO4 HCl Molecular weight 339.81
Cocaine hydrochloride also contains the following inactive ingredients: purified water, citric acid (anhydrous), sodium benzoate, D&C Yellow No. 10, and FD&C Green No. 3.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Cocaine hydrochloride is a local anesthetic of the ester type. Cocaine hydrochloride prevents conduction in nerve fibers by reversibly blocking voltage-gated sodium channels and preventing the transient rise in sodium conductance necessary for generation of an action potential.
12.2 Pharmacodynamics
Cardiac Electrophysiology
The effect of cocaine hydrochloride topical solution on the QTc interval was evaluated in a randomized, positive- and placebo-controlled four-period crossover thorough QTc study in 32 healthy subjects. Cocaine hydrochloride is associated with concentration-dependent QTc prolongation. Based on the concentration-QTc relationship, the mean placebo corrected change from the baseline QTcF (90% two-sided upper confidence interval) are 4.7 ms (6.2 ms) and 15.4 ms (20.1 ms) at peak concentrations of 143 ng/mL (corresponds to 4% single dose, 160 mg) and 434 ng/mL (corresponds to 10% single dose, 400 mg), respectively. The estimates of the QTcF interval are confounded by increased heart rates. The QTc prolongation observed with cocaine hydrochloride (4% single dose) was found to be below the regulatory threshold for concern.
Cocaine hydrochloride is associated with increases in heart rate. The mean placebo corrected change from baseline heart rate (90% two-sided upper confidence interval) are 12 (14) bpm and 20 (22) bpm for the 4% and 10%, respectively.
Effects on Cardiovascular System
Cocaine can potentiate central and peripheral sympathetic effects on the cardiovascular system, resulting in increased inotropic and chronotropic effects, increased cardiac activity, and tachycardia. Intense peripheral vasoconstriction may result in elevated systolic and diastolic blood pressure.
Effects on Vascular System
The relationship between local anesthetic effectiveness and toxicity of cocaine is a function of the patient’s state of health, medical condition, nasal mucosa integrity and extent of systemic absorption of cocaine (from the pledgets). When applied to mucous membranes by pledget administration, topical anesthesia develops rapidly and persists for 30 minutes or longer depending on the concentration of cocaine hydrochloride solution used, the dose, and on the vascularity of the tissue.
12.3 Pharmacokinetics
Cocaine hydrochloride is an aqueous solution of cocaine hydrochloride for topical use only.
Absorption
Application of cocaine hydrochloride for 20 minutes by pledget administration to the nasal mucosa in healthy adults may reduce the systemic absorption of the applied dose of cocaine hydrochloride compared to the systemic absorption after non-pledget administration. The estimated mean systemic absorption of cocaine from a single 160 mg dose (4 mL, 4%) was 23.44% of the topically applied dose. (Table 2).
Table 2. Systemic Absorption of Cocaine Hydrochloride in Healthy Adult Subjects Minimized by Pledget Administration (single nasal dose of 160 mg Cocaine Hydrochloride Topical Solution over 20 minutes)
Cocaine Hydrochloride
Dose (4 mL)Age Range (yr) Application Time
(min)Estimated1 Systemic Absorption Mean Cmax
(ng/mL)
Median
Tmax (min)
Cmax (ng/mL)160 mg (4%) 20-40 20 23.44%
142.68 n=33
30
142.7
1Estimated absorbed dose was calculated by subtracting the residual amount of drug in the pledgets from the administered dose; Tmax includes time 0 (the start of pledget insertion to pledget removal (20 minutes) to the time Cmax was observed, i.e. 10 minutes after removal of the pledgets.
Distribution
Cocaine has been described in literature as approximately 84-92% bound to human plasma proteins. Cocaine is extensively distributed to tissues and crosses the blood brain barrier. Its volume of distribution is approximately 2 L/kg. Cocaine crosses the placenta by simple diffusion and accumulates in the fetus after repeated use.
Elimination
Metabolism
Cocaine is metabolized by two major hydrolytic pathways. Cocaine is metabolized by hydrolysis to benzoylecgonine (major, but inactive metabolite) by hepatic carboxylesterase-1. Cocaine is also metabolized by hydrolysis to ecgonine methyl ester (major, but inactive metabolite) by plasma butyrylcholinesterase and hepatic carboxylesterase-2.
Cocaine is minimally metabolized by hydrolysis to ecgonine (minor, inactive metabolite) by carboxylesterase-2.
Cocaine is N-demethylated by the CYP3A4 enzyme system to produce the active metabolite, norcocaine. Total systemic exposure of norcocaine is less than one percent that observed with cocaine.
Excretion
Cocaine is excreted almost exclusively in the urine, as metabolites. Only a minor fraction of cocaine is eliminated unchanged in the urine (<5%).
The apparent elimination half-life of cocaine following administration of cocaine hydrochloride (by pledgets) was 1.54 hours) for the 4% concentration.
Specific Populations
Geriatric Patients
The pharmacokinetics of cocaine hydrochloride in patients over the age of 65 has not of been studied.
Patients with Hepatic Impairment
The pharmacokinetics of cocaine hydrochloride in patients with hepatic impairment has not been studied.
Patients with Renal Impairment
The pharmacokinetics of cocaine hydrochloride in patients with renal impairment has not been studied.
Drug-drug Interactions:
Disulfiram
It has been reported in the published literature that disulfiram treatment increased plasma cocaine exposure, including both AUC and Cmax, by several folds after acute intranasal cocaine administration. Another published literature reported that co-administration of disulfiram increased AUC of plasma cocaine by several folds after intravenous cocaine administration [see Drug Interactions (7.1)].
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Long-term studies in animals have not been performed to evaluate the carcinogenic potential of cocaine.
Mutagenesis
Cocaine hydrochloride was not mutagenic in an in vitro bacterial reverse mutation assay (Ames test) and was not clastogenic in an in vitro chromosomal aberrations assay or in the in vivo rat micronucleus test.
Impairment of Fertility
Studies in animals to characterize the effects of cocaine on fertility have not been completed. There are published studies that provide some information on the potential impact of cocaine on fertility. Exposure margins below are based on body surface area comparison to the human reference dose (HRD) of 37.5 mg (estimated amount absorbed from the 160 mg cocaine-soaked pledgets).
Suppression of estrous/menstrual cyclicity and ovulation was reported 1.3 to 2.6 times and 2 times the HRD in rats and monkeys, respectively.
In a published study in older male rats (16 weeks) 30 mg/kg cocaine SC (7.8 times the HRD) for 72 days prior to mating did not alter male fertility or alter male reproductive tissue histopathology but did increase the incidence of abnormal sperm and resulted in hyperactivity of next generation offspring.
-
14 CLINICAL STUDIES
Efficacy was demonstrated in one multicenter, randomized, double-blind, placebo-controlled, parallel-arm, clinical trial comparing a single-dose of 4% and 10% cocaine hydrochloride and placebo applied to pledgets and delivered to the nasal mucosa in patients requiring a diagnostic or surgical procedure on or through accessible mucous membranes of the nasal cavities. One trial was terminated early in order to update the trial to refine its operation.
The primary endpoint was nasal anesthetic success, defined as immediate anesthesia based on a numeric pain rating score (NPRS) of 0 (no pain, 0 to 10 scale) 20 minutes post-application of the nasal cavity pledget dose, and sustained anesthesia based on the lack of need for additional anesthesia or analgesics for the remainder of the diagnostic procedure or surgery.
Study drug was applied to the nasal mucosa for 20 minutes via cotton or rayon pledgets, measuring 0.5 inches by 3 inches. The most commonly performed procedures included nasal endoscopy (62%) and transnasal laryngoscopy (24%). Less frequently performed procedures included sinus endoscopy (6%), nasal biopsy (0.25%), and turbinate reduction (0.25%).
Nasal anesthesia was assessed using the visual numeric rating scale (VNRS) during a von Frey monofilament test prior to the diagnostic procedure or surgery. After subject-reported pain scores were collected, the blind to placebo was broken and placebo patients did not undergo a study procedure or surgery. Cocaine patients who reported a pain score of 0 proceeded to the scheduled procedure.
A total of 639 patients were randomized and received a single application of either cocaine hydrochloride nasal solution, 4% (n=258), cocaine hydrochloride nasal solution, 10% (n=254), or placebo (n=127). Sixty-one percent (61%) of randomized patients were female and 81% were white, with a mean age of 38 years (range 18 to 76 years). Patients with a history of myocardial infarction, coronary artery disease, congestive heart failure, irregular heart rhythm, abnormal screening ECG, or uncontrolled hypertension, defined as systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg, were excluded from participating in the study.
The efficacy endpoint was anesthetic success, defined as immediate anesthesia based on a score of 0 on a NPRS during a von Frey monofilament test 20 minutes after study drug administration, and sustained anesthesia throughout the diagnostic procedure or surgery based on no further anesthetic or analgesic treatment required.
Efficacy Results
Table 3 provides results for anesthetic success rate by treatment group.
Table 3: Anesthetic Success
Event Placebo
(N=127)Cocaine 4%
(N=258)Cocaine 10%
(N=254)Success 25 (20%) 183 (71%) 210 (83%) Failure 102 (80%) 75 (29%) 44 (17%) Of the 75 (29%) failures in the cocaine hydrochloride 4% group, 2 patients requested additional anesthetic medication. Of the 44 (17%) failures in the cocaine 10% group, 1 patient requested additional anesthetic medication.
- 16 HOW SUPPLIED/STORAGE AND HANDLING
-
17 PATIENT COUNSELING INFORMATION
Pregnancy
Inform female patients of reproductive potential that cocaine hydrochloride may cause fetal harm and to inform their prescriber of a known or suspected pregnancy [see Use in Specific Populations (8.1)].
Lactation
Advise a woman that breastfeeding is not recommended during treatment with cocaine hydrochloride and to pump and discard breastmilk for 48 hours after administration of cocaine hydrochloride [see Use in Specific Populations (8.2)].
Blood Pressure and Heart Rate Increase
Advise patients that cocaine hydrochloride can cause increases in blood pressure and heart rate. Instruct patients to contact their health care professional if these symptoms persist [see Warnings and Precautions (5.3)].
Seizures
Advise patients that cocaine hydrochloride may lower the seizure threshold. Patients should be monitored for the development of seizures [see Warnings and Precautions (5.2)].
Toxicology Screening
The time after cocaine administration for which cocaine and its metabolites can be detected in plasma and urine depends on the sensitivity of the utilized assay method. Advise patients that cocaine hydrochloride and its metabolites in cocaine hydrochloride may be detected in plasma for up to one week after administration. Cocaine hydrochloride and its metabolites may be detected in urine toxicology screening for longer than one week after administration [see Warnings and Precautions (5.4)].
Concomitant CNS Stimulants
Advise patients to inform their health care professional if they are taking CNS stimulants, alpha-modifying agents, monoamine oxidase inhibitors, or tricyclic antidepressants [see Drug Interactions (7.1)].
Hypersensitivity Reactions
Advise patients of the signs and symptoms of hypersensitivity reactions and to seek immediate medical attention should they occur [see Warnings and Precautions (5.5)].
Distributed by:
Omnivium Pharmaceuticals
Rahway, NJ 07065
CIB72181A
Rev. 08/2023 - 4% 4 mL Vial Label
- 4% 4 mL Carton
-
INGREDIENTS AND APPEARANCE
COCAINE HYDROCHLORIDE NASAL
cocaine hydrochloride nasal solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:81665-301 Route of Administration TOPICAL DEA Schedule CII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength COCAINE HYDROCHLORIDE (UNII: XH8T8T6WZH) (COCAINE - UNII:I5Y540LHVR) COCAINE HYDROCHLORIDE 40 mg in 1 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM BENZOATE (UNII: OJ245FE5EU) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C GREEN NO. 3 (UNII: 3P3ONR6O1S) WATER (UNII: 059QF0KO0R) Product Characteristics Color blue, green Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:81665-301-02 1 in 1 CARTON 10/15/2023 1 4 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA authorized generic NDA209575 10/15/2023 Labeler - OMNIVIUM PHARMACEUTICALS LLC. (117919347) Establishment Name Address ID/FEI Business Operations Lannett Company, Inc. 006422406 analysis(81665-301) , label(81665-301) , manufacture(81665-301) , pack(81665-301)