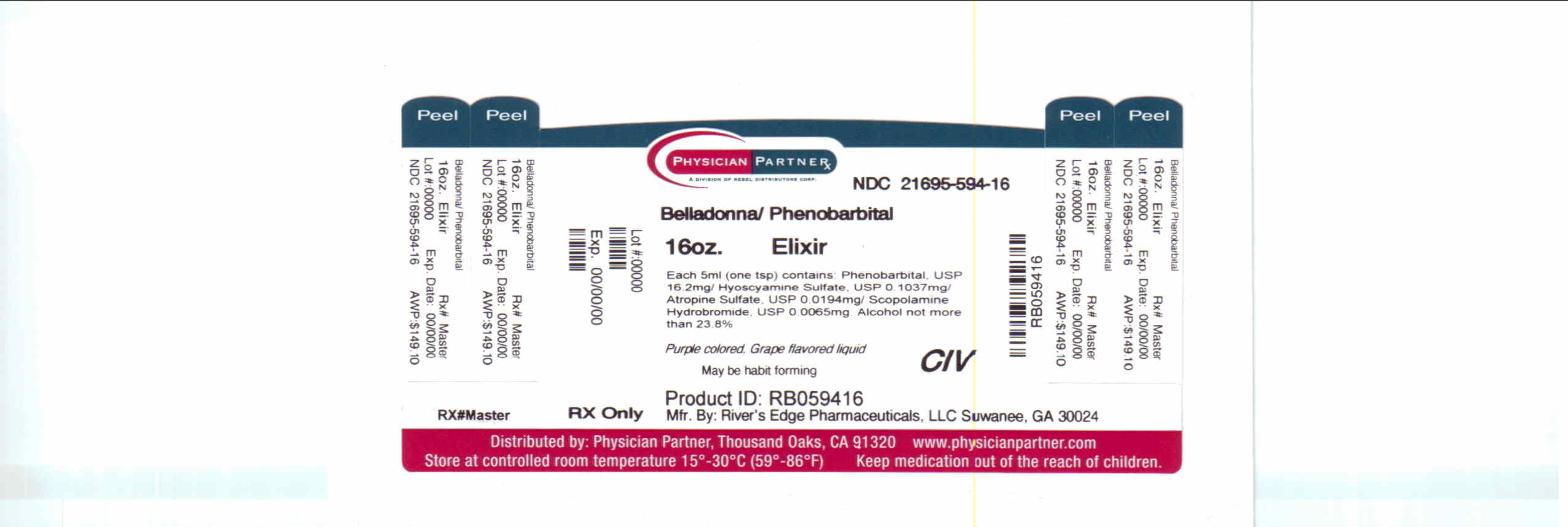
Label: RE-PB HYOS ELIXIR- belladonna alkaloids w/ phenobarbital elixir
-
Contains inactivated NDC Code(s)
NDC Code(s): 21695-594-16 - Packager: Rebel Distributors Corp
- This is a repackaged label.
- Source NDC Code(s): 68032-395
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CIV
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 26, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Each 5 mL (teaspoonful) of elixir contains:
Phenobarbital, USP ............................................. 16.2 mg
(WARNING: may be habit forming)
Hyoscyamine Sulfate, USP .............................. 0.1037 mg
Atropine Sulfate, USP ..................................... 0.0194 mg
Scopolamine Hydrobromide, USP ................... 0.0065 mg
Alcohol not more than 23.8%
INACTIVE INGREDIENTS:
Artificial Grape Flavor, Ethyl Alcohol, FD and C Blue #1, FD and C Red #40, Glycerin, Purified Water USP, Sodium Saccharin,
Sorbitol Solution 70%, and Sucrose.
- CLINICAL PHARMACOLOGY
-
INDICATION AND USAGE
FDA has classified the following indications as “possibly” effective: For use as adjunctive therapy in the treatment of
irritable bowel syndrome (irritable colon, spastic colon, mucous colitis) and acute enterocolitis. May also be useful as
adjunctive therapy in the treatment of duodenal ulcer.
IT HAS NOT BEEN SHOWN CONCLUSIVELY WHETHER ANTICHOLINERGIC/ ANTISPASMODIC DRUGS AID IN THE
HEALING OF A DUODENAL ULCER, DECREASE THE RATE OF RECURRENCES OR PREVENT COMPLICATIONS.
-
CONTRAINDICATIONS
RE-PB Hyos Elixir is contraindicated in patients with known hypersensitivity to any of the ingredients. Phenobarbital is
contraindicated in patients with acute intermittent porphyria and in those patients in which phenobarbital produces
restlessness and/or excitement.It is also contraindicated in patients with glaucoma, obstructive uropathy; paralytic ileus; myasthenia gravis;
intestinal atony; unstable cardiovascular status in acute hemorrhage; hiatal hernia associated with reflux esophagitis;
obstructive disease of the gastrointestinal tract; or severe ulcerative colitis
-
WARNINGS
Heat prostration can occur with belladonna alkaloids in high temperatures.
Diarrhea may be an early symptom of incomplete intestinal obstruction, especially in patients with ileostomy or colostomy.
In this instance, treatment with this drug could be harmful.RE-PB Hyos Elixir may produce drowsiness and blurred vision. The patient should be warned about engaging in hazardous work or activities requiring mental alertness, such as operating a motor vehicle or other machinery. Phenobarbital may decrease the effect of anticoagulants, and larger doses of the anticoagulant may be needed for optimal effect. When phenobarbital is discontinued, the dose of the anticoagulant may have to be decreased. Phenobarbital may be habit forming and should not be administered to patients who are susceptible to addiction or to those with a history of physical and/or psychological drug dependence.
Barbiturates should be used with caution in patients with hepatic dysfunction.
-
PRECAUTIONS
GENERAL:
Use with caution in patients with: autonomic neuropathy, hepatic or renal disease, hyperthyroidism, coronary heart
disease, congestive heart failure, cardiac arrhythmias, tachycardia, and hypertension.
Belladonna alkaloids may produce a delay in gastric emptying (antral stasis) which would complicate the management of
gastric ulcer. Do not rely on the use of the drug in the presence of complication of biliary tract disease. Theoretically,
with overdosage, a curare-like action may occur.CARCINOGENESIS, MUTAGENESIS, IMPAIRMENT OF FERTILITY:
Long-term studies in animals have not been performed to evaluate carcinogenic potential.PREGNANCY CATEGORY C:
Animal reproduction studies have not been conducted with RE-PB Hyos Elixir. It is not known whether RE-PB Hyos Elixir
can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. RE-PB Hyos Elixir
should be given to a pregnant woman only if clearly needed.
NURSING MOTHERS:
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution
should be exercised when RE-PB Hyos Elixir is administered to a nursing woman.
-
ADVERSE REACTIONS
Adverse reactions associated with anticholinergics and/or anticonvulsants are: dry mouth; tachycardia; urinary
hesitancy and retention; palpitation; blurred vision; prolonged pupil dilation; cycloplegia; increased ocular tension; loss of
taste sense; headache; nervousness; drowsiness; weakness; dizziness; insomnia; nausea; vomiting; severe allergic
reaction or drug idiosyncrasies, including anaphylaxis, hives and/or other dermal manifestations; decreased sweating; impotence; suppression of lactation; constipation; bloated feeling and musculoskeletal pain. Elderly patients may react
with symptoms of excitement, agitation and drowsiness to even small doses of the drug. Phenobarbital may produce
excitement in some patients, rather than a sedative effect. In patients habituated to barbiturates, abrupt withdrawal may
produce delirium or convulsions.Call your doctor for medical advice about side effects. You may report side effects to the FDA at 1-800-FDA-1088.
- OVERDOSAGE
-
DOSAGE & ADMINISTRATION
The dosage of RE-PB Hyos Elixir should be adjusted to the needs of the individual patient to assure
symptomatic control with a minimum of adverse effects.Adults: One or two teaspoonfuls of elixir three or four times a day according to conditions and severity of symptoms.
Pediatric patients: may be dosed every 4 to 6 hours.
Starting Dosage
Body Weight
q4h q6h 10 lb. (4.5 kg)
0.5 mL
0.75 mL
20 lb. (9.1 kg)
1.0 mL
1.5 mL
30 lb. (13.6 kg)
1.5 mL
2.0 mL
50 lb. (22.7 kg)
1/2 tsp
3/4 tsp
75 lb. (34 kg)
3/4 tsp
1 tsp
100 lb. (45.4kg)
1 tsp
1 1/2 tsp
-
HOW SUPPLIED
RE-PB Hyos Elixir is a purple colored, grape flavored liquid.
NDC 21695-594-16 in bottles of 16 oz.
WARNINGS: KEEP THIS AND ALL DRUGS OUT OF THE REACH OF CHILDREN. IN CASE OF ACCIDENTAL
OVERDOSE, SEEK PROFESSIONAL ASSISTANCE OR CONTACT A POISON CONTROL CENTER IMMEDIATELY.
Manufactured by:
Great Southern Laboratories
Houston, TX 77099-3405Manufactured for:
River’s Edge Pharmaceuticals, LLC
Suwanee, GA 30024Repackaged by:
Rebel Distributors CorpThousand Oaks, CA 91320
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
RE-PB HYOS ELIXIR
belladonna alkaloids w/ phenobarbital elixirProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:21695-594(NDC:68032-395) Route of Administration ORAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PHENOBARBITAL (UNII: YQE403BP4D) (PHENOBARBITAL - UNII:YQE403BP4D) PHENOBARBITAL 16.2 mg in 5 mL HYOSCYAMINE SULFATE (UNII: F2R8V82B84) (HYOSCYAMINE - UNII:PX44XO846X) HYOSCYAMINE SULFATE 0.1037 mg in 5 mL ATROPINE SULFATE (UNII: 03J5ZE7KA5) (ATROPINE - UNII:7C0697DR9I) ATROPINE SULFATE 0.0194 mg in 5 mL SCOPOLAMINE HYDROBROMIDE (UNII: 451IFR0GXB) (SCOPOLAMINE - UNII:DL48G20X8X) SCOPOLAMINE HYDROBROMIDE 0.0065 mg in 5 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) FD&C RED NO. 40 (UNII: WZB9127XOA) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) SACCHARIN SODIUM (UNII: SB8ZUX40TY) SORBITOL (UNII: 506T60A25R) SUCROSE (UNII: C151H8M554) Product Characteristics Color purple Score Shape Size Flavor GRAPE Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:21695-594-16 480 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug other 10/20/2009 Labeler - Rebel Distributors Corp (118802834) Establishment Name Address ID/FEI Business Operations Rebel Distributors Corp 118802834 RELABEL, REPACK