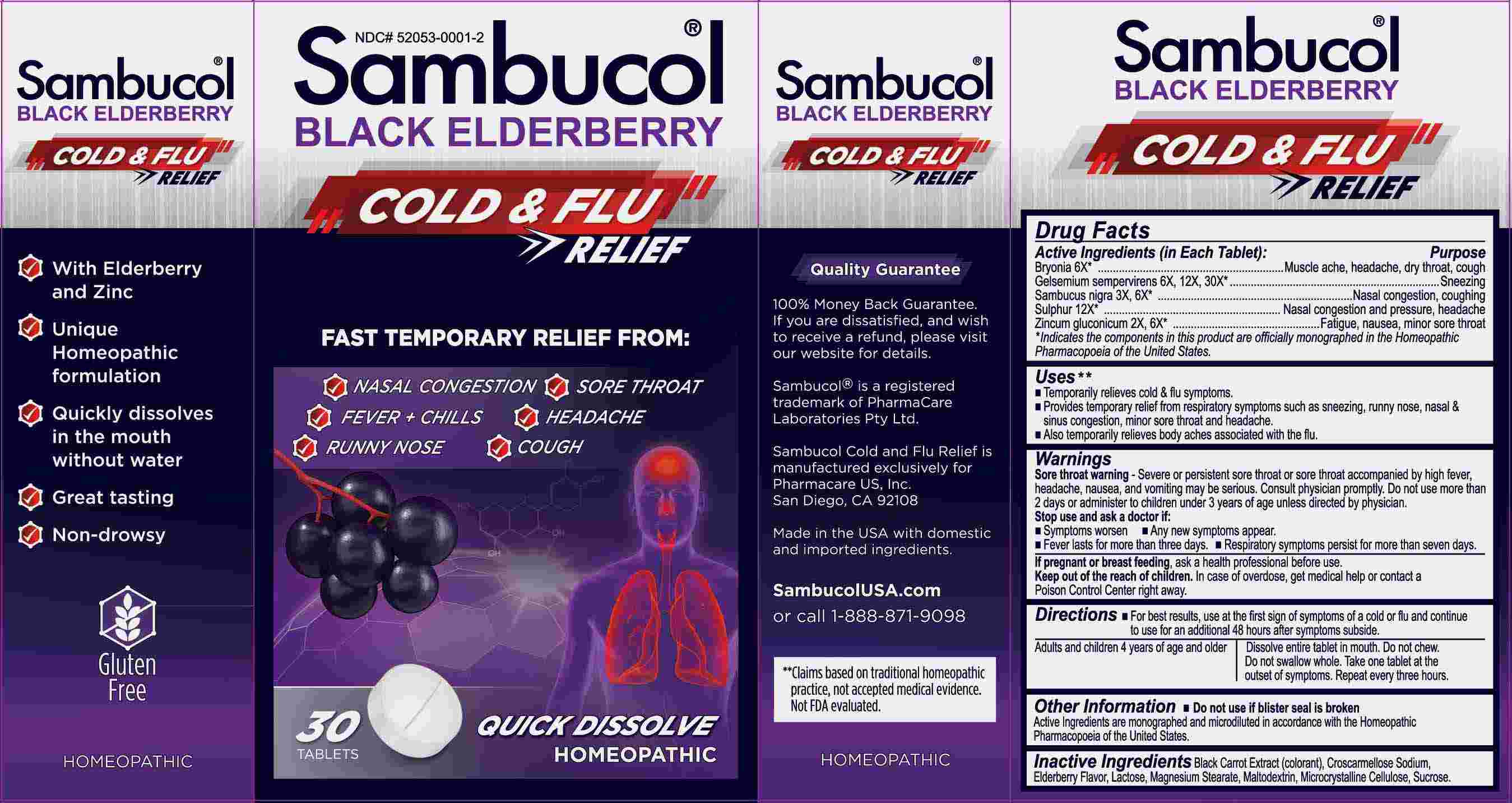
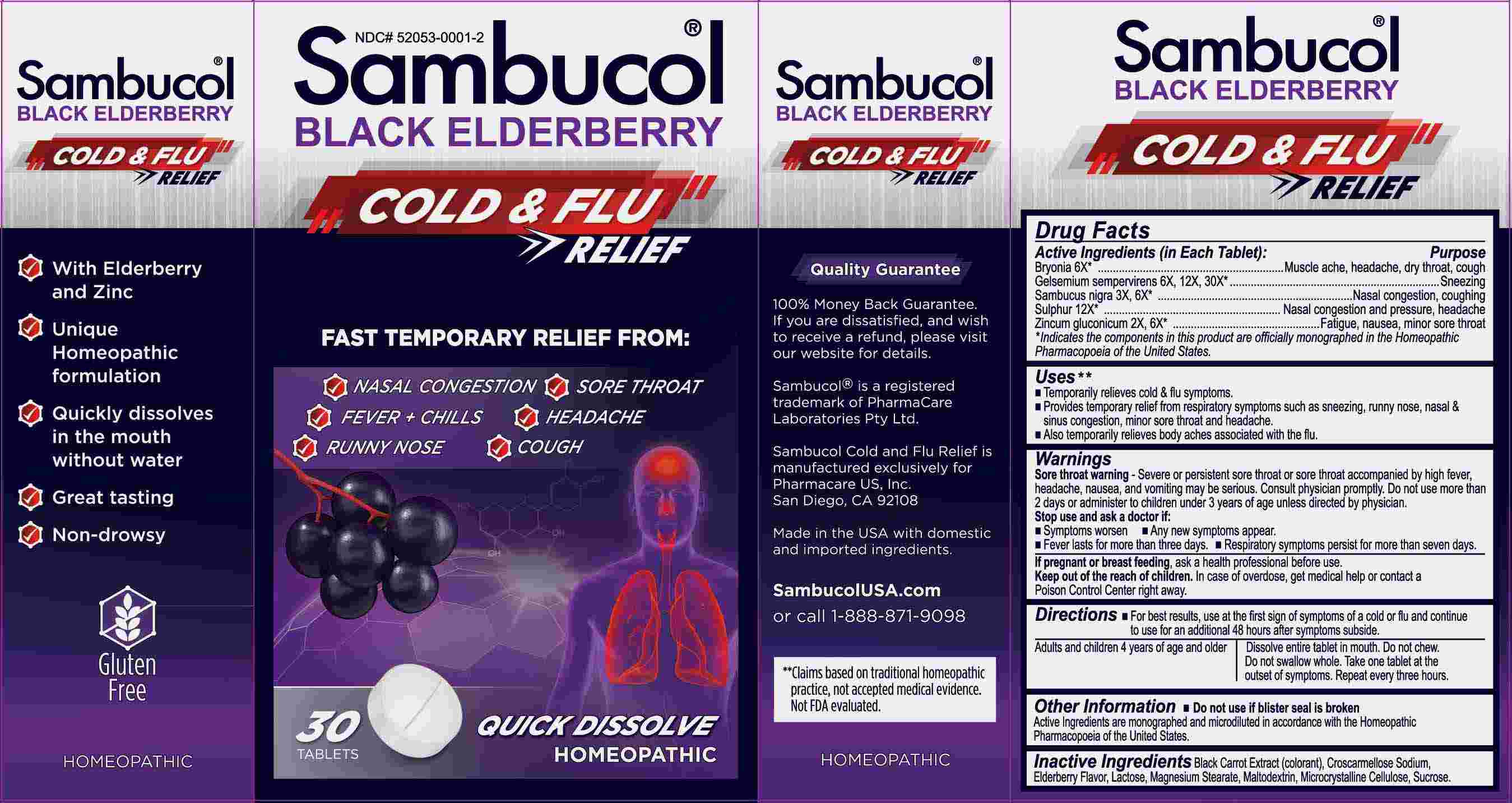
Label: SAMBUCOL COLD AND FLU RELIEF- bryonia, gelsemium sempervirens, sabucus nigra, sulphur, zincum gluconicum tablet, orally disintegrating
- NDC Code(s): 52053-0001-1, 52053-0001-2
- Packager: PharmaCare US, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated August 15, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENTS:
-
PURPOSE:
Bryonia – Muscle ache, headache, dry throat, cough,* Gelsemium Sempervirens - Sneezing,* Sambucus Nigra – Nasal congestion, coughing,* Sulphur – Nasal congestion and pressure, headache,* Zincum Gluconicum – Fatigue, nausea, minor sore throat.*
*Indicates the components in this product are officially monographed in the Homeopathic Pharmacopoeia of the United States. -
USES:
• Temporarily relieves cold & flu symptoms.**
• Provides temporary relief from respiratory symptoms such as sneezing, runny nose, nasal & sinus congestion, minor sore throat and
headache.**
• Also temporarily relieves body aches associated with the flu.**
**Claims based on traditional homeopathic practice, not accepted medical evidence. Not FDA evaluated.
-
WARNINGS:
Sore throat warning - Severe or persistent sore throat or sore throat accompanied by high fever, headache, nausea, and vomiting may be serious. Consult physician promptly. Do not use more than 2 days or administer to children under 3 years of age unless directed by a physician. Stop use and ask a doctor if:
• Symptoms worsen • Any new symptoms appear.
• Fever lasts for more than three days.
• Respiratory symptoms persist for more than seven days.
If pregnant or breast feeding, ask a health professional before using.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Do not use if blister seal is broken.
Active Ingredients are monographed and microdiluted in accordance with the Homeopathic Pharmacopoeia of the United States.
- KEEP OUT OF REACH OF CHILDREN:
-
DIRECTIONS:
• For best results, use at the first sign of symptoms of a cold or flu and continue to use for an additional 48 hours after symptoms subside.
Adults and children 4 years of age and older. Dissolve entire tablet in mouth. Do not chew. Do not swallow whole. Take one tablet at the outset of symptoms. Repeat every three hours.
- INACTIVE INGREDIENTS:
- QUESTIONS:
- PACKAGE LABEL DISPLAY:
-
INGREDIENTS AND APPEARANCE
SAMBUCOL COLD AND FLU RELIEF
bryonia, gelsemium sempervirens, sabucus nigra, sulphur, zincum gluconicum tablet, orally disintegratingProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:52053-0001 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BRYONIA ALBA ROOT (UNII: T7J046YI2B) (BRYONIA ALBA ROOT - UNII:T7J046YI2B) BRYONIA ALBA ROOT 6 [hp_X] in 1 mg GELSEMIUM SEMPERVIRENS ROOT (UNII: 639KR60Q1Q) (GELSEMIUM SEMPERVIRENS ROOT - UNII:639KR60Q1Q) GELSEMIUM SEMPERVIRENS ROOT 6 [hp_X] in 1 mg SAMBUCUS NIGRA FLOWERING TOP (UNII: CT03BSA18U) (SAMBUCUS NIGRA FLOWERING TOP - UNII:CT03BSA18U) SAMBUCUS NIGRA FLOWERING TOP 3 [hp_X] in 1 mg SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 12 [hp_X] in 1 mg ZINC GLUCONATE (UNII: U6WSN5SQ1Z) (ZINC CATION - UNII:13S1S8SF37) ZINC GLUCONATE 2 [hp_X] in 1 mg Inactive Ingredients Ingredient Name Strength BLACK CARROT ANTHOCYANINS (UNII: 971DA56IOL) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) LACTOSE (UNII: J2B2A4N98G) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) SUCROSE (UNII: C151H8M554) EUROPEAN ELDERBERRY JUICE (UNII: Z4IFJ0AK1E) Product Characteristics Color purple (off purple off grey color with specks) Score no score Shape ROUND Size 10mm Flavor BERRY (Elderberry) Imprint Code SCF Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52053-0001-2 2 in 1 CARTON 06/30/2017 1 NDC:52053-0001-1 4500 mg in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 03/26/2013 Labeler - PharmaCare US, Inc. (026889965) Registrant - Apotheca Company (844330915) Establishment Name Address ID/FEI Business Operations Apotheca Company 844330915 manufacture(52053-0001) , api manufacture(52053-0001) , label(52053-0001) , pack(52053-0001)