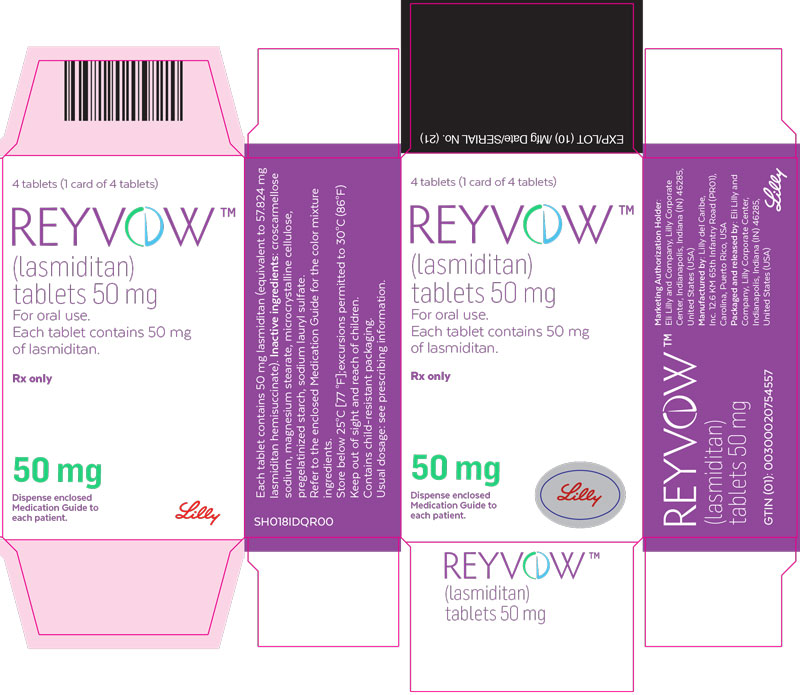
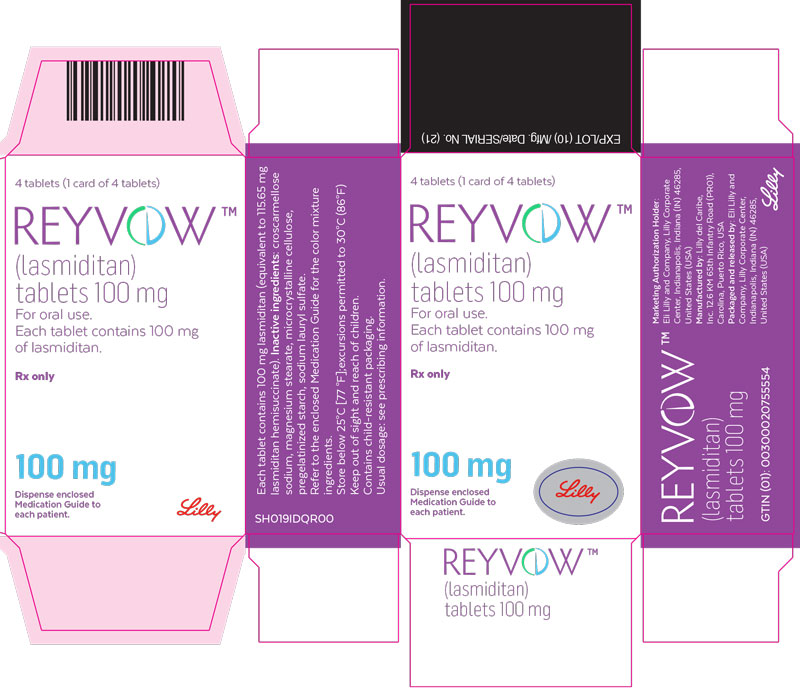
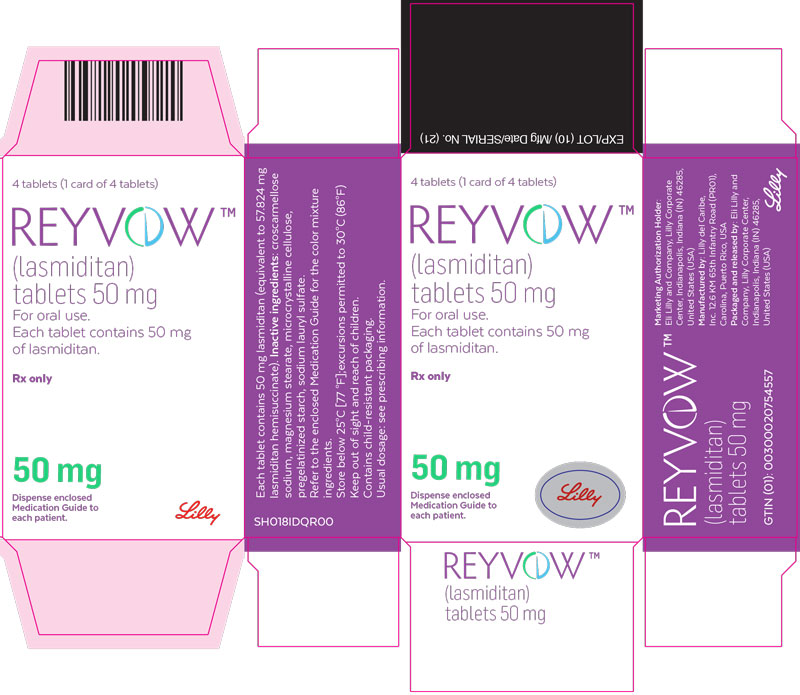
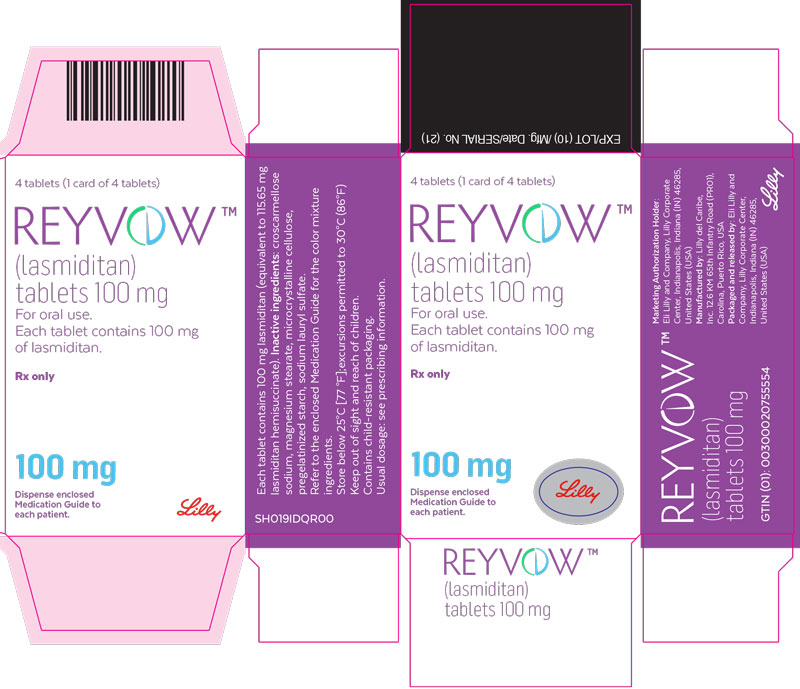
Label: REYVOW- lasmiditan tablet
- NDC Code(s): 0002-0265-04, 0002-0632-04
- Packager: Eli Lilly and Company
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CV
- Marketing Status: Export only
Drug Label Information
Updated January 9, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
REYVOW
lasmiditan tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0002-0265 Route of Administration ORAL DEA Schedule CV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength lasmiditan (UNII: 760I9WM792) (lasmiditan - UNII:760I9WM792) lasmiditan 50 mg Inactive Ingredients Ingredient Name Strength Microcrystalline cellulose (UNII: OP1R32D61U) Starch, corn (UNII: O8232NY3SJ) Croscarmellose Sodium (UNII: M28OL1HH48) Sodium Lauryl Sulfate (UNII: 368GB5141J) Magnesium Stearate (UNII: 70097M6I30) Polyvinyl Alcohol, Unspecified (UNII: 532B59J990) Titanium dioxide (UNII: 15FIX9V2JP) Polyethylene Glycol 3350 (UNII: G2M7P15E5P) Talc (UNII: 7SEV7J4R1U) Ferrosoferric Oxide (UNII: XM0M87F357) Product Characteristics Color gray (light gray) Score no score Shape OVAL (oval) Size 9mm Flavor Imprint Code L50;4312 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0002-0265-04 1 in 1 CARTON 08/21/2020 1 4 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 08/21/2020 REYVOW
lasmiditan tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0002-0632 Route of Administration ORAL DEA Schedule CV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength lasmiditan (UNII: 760I9WM792) (lasmiditan - UNII:760I9WM792) lasmiditan 100 mg Inactive Ingredients Ingredient Name Strength Microcrystalline cellulose (UNII: OP1R32D61U) Starch, corn (UNII: O8232NY3SJ) Croscarmellose Sodium (UNII: M28OL1HH48) Sodium Lauryl Sulfate (UNII: 368GB5141J) Magnesium Stearate (UNII: 70097M6I30) Polyvinyl Alcohol, Unspecified (UNII: 532B59J990) Titanium dioxide (UNII: 15FIX9V2JP) Polyethylene Glycol 3350 (UNII: G2M7P15E5P) Talc (UNII: 7SEV7J4R1U) Ferrosoferric Oxide (UNII: XM0M87F357) Ferric Oxide Red (UNII: 1K09F3G675) Product Characteristics Color purple (light purple) Score no score Shape OVAL Size 12mm Flavor Imprint Code L100;4491 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0002-0632-04 1 in 1 CARTON 08/21/2020 1 4 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 08/21/2020 Labeler - Eli Lilly and Company (006421325)