Label: NAPHCON A- naphazoline hydrochloride and pheniramine maleate solution/ drops
- NDC Code(s): 0065-0085-15, 0065-0085-38, 0065-0085-42, 0065-0085-52
- Packager: Alcon Laboratories, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated March 27, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- Uses
- Warnings
- Do not use
- Ask a doctor before use if you have
- When using this product
- Stop use and ask a doctor if
- Keep out of the reach of children.
- Directions
- Other information
- Inactive ingredients
- Questions?
-
PRINCIPAL DISPLAY PANEL
$3 Coupon Inside
NAPHCON A®
Naphazoline Hydrochloride 0.025% and Pheniramine Maleate 0.3%
Ophthalmic Solution
Redness Reliever and Antihistamine
RELIEVES ITCHING & REDNESS
EYE DROPS
STERILE 15 mL (0.5 FL OZ)
TAMPER EVIDENT: For your protection, this bottle has a seal imprinted with the word “Alcon” around the neck. Do not use if seal is damaged or missing at time of purchase.
Actual Size
Combines a redness reliever and an antihistamine for the temporary relief of itchy, red eyes.
SEE COUPON INSIDE
ALCON LABORATORIES, INC.
6201 South Freeway
Fort Worth, TX 76134 USA
Alcon
LOT:
EXP.:
300055595-0122

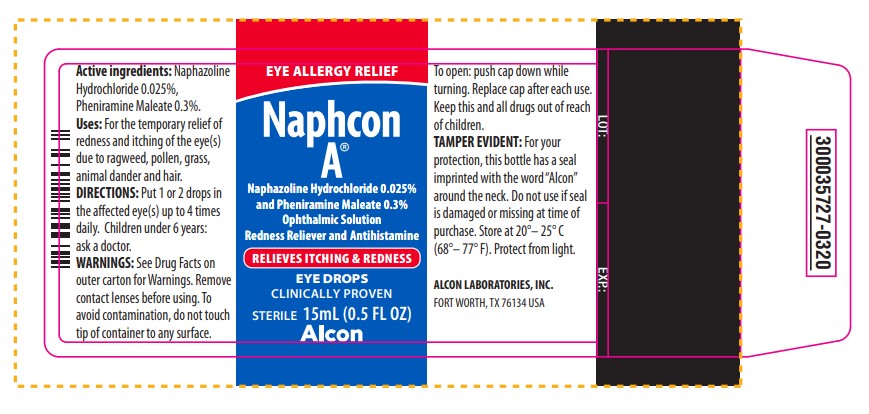
EYE ALLERGY RELIEF
NAPHCON A®
Naphazoline Hydrochloride 0.025% and Pheniramine Maleate 0.3%
Ophthalmic Solution
Redness Reliever and Antihistamine
RELIEVES ITCHING & REDNESS
EYE DROPS
CLINICALLY PROVEN
STERILE 15 mL (0.5 FL OZ)
Alcon
Active ingredients: Naphazoline
Active ingredients: Naphazoline Hydrochloride 0.025%, Pheniramine Maleate 0.3%.
Uses: For the temporary relief of redness and itching of the eye(s) due to ragweed, pollen, grass, animal dander and hair.
DIRECTIONS: Put 1 or 2 drops in the affected eye(s) up to 4 times daily. Children under 6 years: ask a doctor.
WARNINGS: See Drug Facts on outer carton for Warnings. Remove contact lenses before using. To avoid contamination, do not touch tip of container to any surface.
To open: push cap down while turning. Replace cap after each use.
Keep this and all drugs out of reach of children.
TAMPER EVIDENT: For your protection, this bottle has a seal imprinted with the word “Alcon” around the neck. Do not use if seal is damaged or missing at time of purchase. Store at 20° - 25°C (68° - 77°F). Protect from light.
ALCON LABORATORIES, INC.
FORT WORTH, TX 76134 USA
LOT: EXP:
300035727-0320
-
PRINCIPAL DISPLAY PANEL
Convenient • Take Anywhere
EYE ALLERGY RELIEF
POCKET SIZE
NAPHCON A®
Naphazoline Hydrochloride 0.025% and Pheniramine Maleate 0.3%
Ophthalmic Solution
Redness Reliever and Antihistamine
Relieves Itching & Redness
EYE DROPS
CLINICALLY PROVEN
STERILE Two 5 mL (0.17 FL OZ) Bottles
TAMPER EVIDENT: For your protection, this bottle has a seal imprinted with the word “Alcon” around the neck. Do not use if seal is damaged or missing at time of purchase.
ACTUAL SIZE
Combines a redness reliever and an antihistamine for the temporary relief of itchy, red eyes.
ACTUAL FILL
ALCON LABORATORIES, INC.
6201 South Freeway
Fort Worth, TX 76134
ALCON
LOT:
EXP.:
9017143-0419

-
PRINCIPAL DISPLAY PANEL
EYE ALLERGY RELIEF
NAPHCON A®
Naphazoline Hydrochloride 0.025% and Pheniramine Maleate 0.3% Ophthalmic Solution
Redness Reliever and Antihistamine
RELIEVES ITCHING & REDNESS
EYE DROPS
CLINICALLY PROVEN
STERILE 5mL (0.17 FL OZ )
Active ingredients: Naphazoline Hydrochloride 0.025%, Pheniramine Maleate 0.3%.
Uses: For the temporary relief of redness and itching of the eye(s) due to ragweed, pollen, grass, animal dander and hair.
DIRECTIONS: Put 1 or 2 drops in the affected eye(s) up to 4 times daily. Children under 6 years: ask a doctor.
WARNINGS: See Drug Facts on outer carton for Warnings. Remove contact lenses before using. To avoid contamination, do not touch tip of container to any surface. To open: push cap down while turning. Replace cap after each use. Keep this and all drugs out of reach of children.
TAMPER EVIDENT: For your protection, this bottle has a seal imprinted with the word “Alcon” around the neck. Do not use if seal is damaged or missing at time of purchase.
Store at 20° - 25°C (68° - 77°F). Protect from light.
ALCON LABORATORIES, INC.
FORT WORTH, TX 76134 USA
LOT
EXP:
300035726-0320

-
INGREDIENTS AND APPEARANCE
NAPHCON A
naphazoline hydrochloride and pheniramine maleate solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0065-0085 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Naphazoline Hydrochloride (UNII: MZ1131787D) (Naphazoline - UNII:H231GF11BV) Naphazoline Hydrochloride 0.25 mg in 1 mL Pheniramine Maleate (UNII: NYW905655B) (Pheniramine - UNII:134FM9ZZ6M) Pheniramine Maleate 3 mg in 1 mL Inactive Ingredients Ingredient Name Strength Benzalkonium Chloride (UNII: F5UM2KM3W7) Boric Acid (UNII: R57ZHV85D4) Edetate Disodium (UNII: 7FLD91C86K) Water (UNII: 059QF0KO0R) Sodium Borate (UNII: 91MBZ8H3QO) Sodium Chloride (UNII: 451W47IQ8X) Sodium Hydroxide (UNII: 55X04QC32I) Hydrochloric Acid (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0065-0085-15 1 in 1 CARTON 01/03/1995 1 15 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:0065-0085-38 1 in 1 CARTON 06/08/1994 02/28/2019 2 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 3 NDC:0065-0085-42 2 in 1 CARTON 01/07/2005 03/31/2024 3 5 mL in 1 BOTTLE; Type 0: Not a Combination Product 4 NDC:0065-0085-52 1 in 1 CARTON 06/08/1994 11/30/2021 4 5 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020226 06/08/1994 Labeler - Alcon Laboratories, Inc. (008018525) Registrant - Alcon Laboratories, Inc. (008018525) Establishment Name Address ID/FEI Business Operations Alcon Research LLC 007672236 manufacture(0065-0085) Establishment Name Address ID/FEI Business Operations Loba biotech GmbH 300137478 api manufacture(0065-0085) Establishment Name Address ID/FEI Business Operations Supriya Lifescience Limited 650542744 api manufacture(0065-0085) Establishment Name Address ID/FEI Business Operations Kongo Chemical Co., Ltd. 739918627 api manufacture(0065-0085)