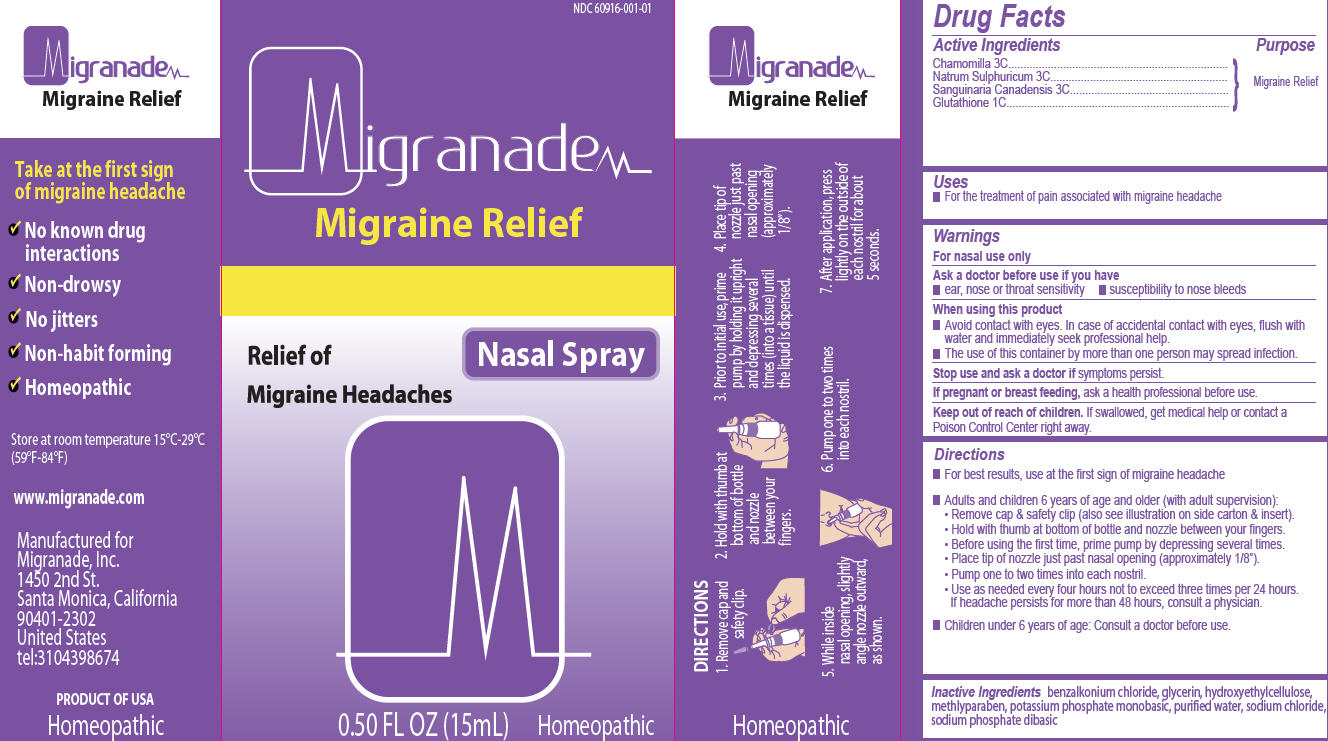
Label: MIGRANADE- chamomilla, natrum sulphuricum, sanguinaria canadensis, glutathione spray
-
Contains inactivated NDC Code(s)
NDC Code(s): 60916-001-01 - Packager: Migranade Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated January 22, 2014
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Uses
- Warnings
- Ask a doctor before use if you have
- When using this product
- Stop use and ask a doctor
- If pregnant or breast feeding,
- Keep out of reach of children.
-
Directions
- •
- For best results, use at the first sign of migraine headache
- •
- Adults and children 6 years of age and older (with adult supervision):
- o
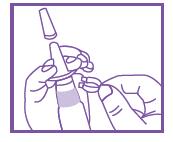
- Remove cap & safety clip (also see illustration on side carton & insert).
- o
- Hold with thumb at bottom of bottle and nozzle between your fingers.
- o
- Before using the first time, prime pump by depressing several times.
- o
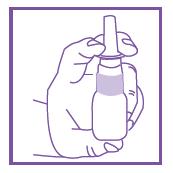
- Place tip o nozzle just past nasal opening (approximately 1/8”).
- o
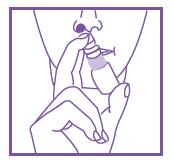
- Pump one to two times into each nostril.
- o
- Use as needed every four hours not exceed three times per 24 hours. If headache persists for more than 48 hours, consult a physician.
- •
- Children under 6 years of age: Consult a doctor before use.
- Inactive Ingredients
-
SPL UNCLASSIFIED SECTION
Take at the first sign of migraine headache
-
- No known drug interactions
-
- Non-drowsy
-
- No jitters
-
- Non-habit forming
-
- Homeopathic
Store at room temperature 15°C-29C-29°C (59°F-84°F)
www.migranade.com
Manufactured for Migranade, Inc.
1450 2nd St.
Santa Monica, California 90401-2302
United States
tel: 3104398674Product of USA Homeopathic
-
Patient Information
Migranade Migraine Relief
Non-DrowsyRelief of Migraine Headache
Homeopathic
DIRECTIONS: Adults and children 6 years of age and older (with adult supervision):
Children under 6 years of age: Consult a doctor before use.
Active Ingredients:
Chamomilla 3C,
Natrum Sulphuricum 3C,
Sanguinaria Canadensis 3C,
Glutathione 1CInactive Ingredients:
benzalkonium chloride, glycerin, hydroxyethylcellulose, methlyparaben, potassium phosphate monobasic, purified water, sodium chloride, sodium phosphate dibasicWarnings:
For nasal use only
Ask a doctor before use if you have-
- ear, nose or throat sensitivity
-
- susceptibility to nose bleeds
When using this product
-
- Avoid contact with eyes. In case of accidental contact with eyes, flush with water and immediately seek professional help.
-
- The use of this container by more than one person may spread infection.
Stop use and ask a doctor if symptoms persist.
If pregnant or breast feeding, ask a health professional before use.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Take at the first sign of migraine headache.
Migranade Migraine Relief
- •
- No known drug interactions
- •
- Non-drowsy
- •
- No jitters
- •
- Non-habit forming
- •
- Homeopathic
Product is naturally dark in color.
- www.migranade.com
Manufactured for:
Migranade Inc.
Santa Monica, California
90401-2302
United States
tel:3104398674- PRODUCT OF USA
- Homeopathic
- Package/Label Principal Display Panel
-
INGREDIENTS AND APPEARANCE
MIGRANADE
chamomilla, natrum sulphuricum, sanguinaria canadensis, glutathione sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:60916-001 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHAMOMILE (UNII: FGL3685T2X) (CHAMOMILE - UNII:FGL3685T2X) CHAMOMILE 3 [hp_C] in 15 mL SODIUM SULFATE (UNII: 0YPR65R21J) (SODIUM SULFATE ANHYDROUS - UNII:36KCS0R750) SODIUM SULFATE 3 [hp_C] in 15 mL SANGUINARIA CANADENSIS ROOT (UNII: N9288CD508) (SANGUINARIA CANADENSIS ROOT - UNII:N9288CD508) SANGUINARIA CANADENSIS ROOT 3 [hp_C] in 15 mL GLUTATHIONE (UNII: GAN16C9B8O) (GLUTATHIONE - UNII:GAN16C9B8O) GLUTATHIONE 1 [hp_C] in 15 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) GLYCERIN (UNII: PDC6A3C0OX) HYDROXYETHYL CELLULOSE (100 MPA.S AT 2%) (UNII: R33S7TK2EP) METHYLPARABEN (UNII: A2I8C7HI9T) POTASSIUM PHOSPHATE, MONOBASIC (UNII: 4J9FJ0HL51) WATER (UNII: 059QF0KO0R) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:60916-001-01 1 in 1 CARTON 1 15 mL in 1 BOTTLE, SPRAY Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved homeopathic 01/27/2014 Labeler - Migranade Inc. (079159028) Establishment Name Address ID/FEI Business Operations Oasis Health Products, Inc. 964858315 MANUFACTURE(60916-001)