Label: COCCIAID- amprolium solution
- NDC Code(s): 51072-090-00
- Packager: Aurora Pharmaceutical, Inc.
- Category: OTC ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Animal Drug Application
Drug Label Information
Updated December 3, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredient:
-
INDICATIONS:
CocciAid® (amprolium) 9.6% Oral Solution is intended for the treatment of coccidiosis in growing chickens, turkeys, and laying hens. If no improvement is noted within 3 days, have the diagnosis confirmed and follow the instructions of your veterinarian or poultry pathologist. Losses may result from intercurrent disease or other conditions affecting drug intake which can contribute to the virulence of coccidiosis under field conditions.
-
USE DIRECTIONS:
Give amprolium at the 0.012% level (8 fl oz per 50 gallons) as soon as coccidiosis is diagnosed and continue for 3 to 5 days. (In severe outbreaks, give amprolium at the 0.024% level.) Continue with 0.006% amprolium medicated water for an additional 1 to 2 weeks. No other source of drinking water should be available to the birds during this time. Use as the sole source of amprolium.
- WARNING:
- PRECAUTIONS:
- STORAGE:
- SPL UNCLASSIFIED SECTION
- QUESTIONS
-
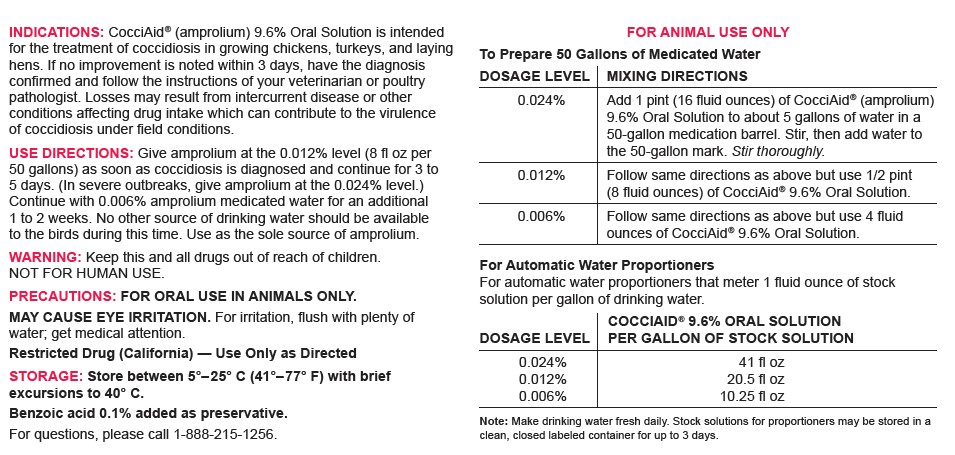
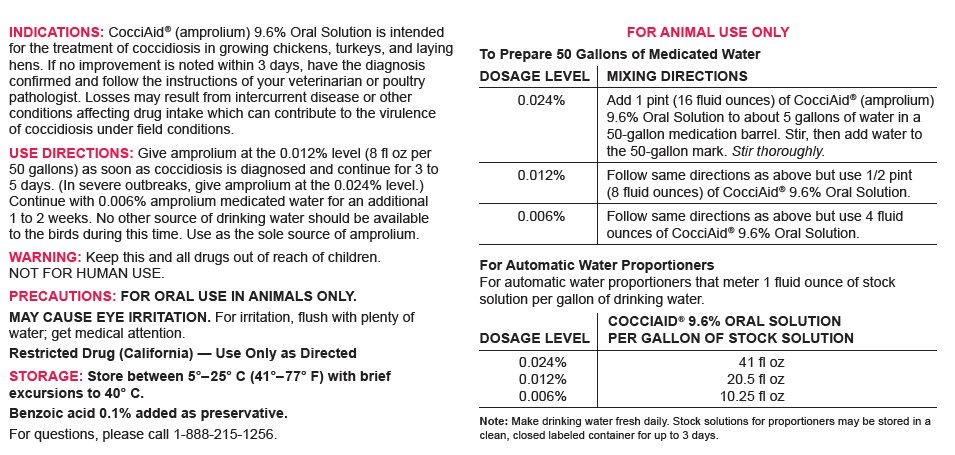
DOSAGE & ADMINISTRATION
FOR ANIMAL USE ONLY
To Prepare 50 Gallons of Medicated WaterDOSAGE LEVEL MIXING DIRECTIONS 0.024% Add 1 pint (16 fluid ounces) of CocciAid® (amprolium) 9.6% Oral Solution to about 5 gallons of water in a 50-gallon medication barrel. Stir, then add water to the 50-gallon mark. Stir thoroughly. 0.012% Follow same directions as above but use 1/2 pint (8 fluid ounces) of CocciAid® 9.6% Oral Solution. 0.006% Follow same directions as above but use 4 fluid ounces of CocciAid® 9.6% Oral Solution. For Automatic Water Proportioners
For automatic water proportioners that meter 1 fluid ounce of stock solution per gallon of drinking water.DOSAGE LEVEL COCCIAID® 9.6% ORAL SOLUTION
PER GALLON OF STOCK SOLUTION0.024%
0.012%
0.006%41 fl oz
20.5 fl oz
10.25 fl ozNote: Make drinking water fresh daily. Stock solutions for proportioners may be stored in a clean, closed labeled container for up to 3 days.
- SPL UNCLASSIFIED SECTION
- Container Label
-
INGREDIENTS AND APPEARANCE
COCCIAID
amprolium solutionProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:51072-090 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AMPROLIUM (UNII: 95CO6N199Q) (AMPROLIUM ION - UNII:H2T307KMZR) AMPROLIUM 96 in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51072-090-00 3785 mL in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200630 08/23/2018 Labeler - Aurora Pharmaceutical, Inc. (832848639) Establishment Name Address ID/FEI Business Operations Aurora Pharmaceutical, Inc. 832848639 manufacture