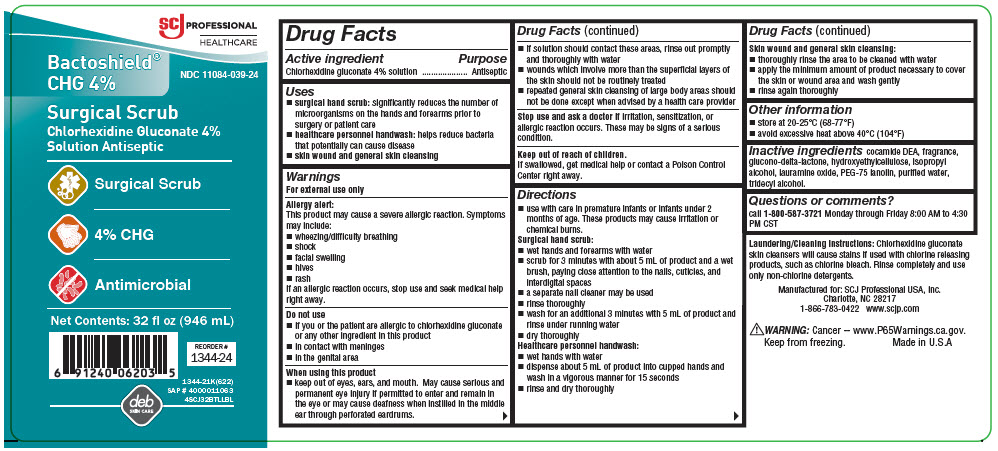
Label: BACTOSHIELD CHG- chlorhexidine gluconate solution
- NDC Code(s): 11084-039-03, 11084-039-24
- Packager: SC Johnson Professional USA, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated November 28, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
-
Warnings
For external use only
Allergy alert
This product may cause a severe allergic reaction. Symptoms may include:
- wheezing/difficulty breathing
- shock
- facial swelling
- hives
- rash
If an allergic reaction occurs, stop use and seek medical help right away.
Do not use
- if you or the patient are allergic to chlorhexidine gluconate or any other ingredient in this product
- in contact with meninges
- in the genital area
When using this product
- keep out of eyes, ears, and mouth. May cause serious and permanent eye injury if permitted to enter and remain in the eye or may cause deafness when instilled in the middle ear through perforated eardrums.
- if solution should contact these areas, rinse out promptly and thoroughly with water
- wounds which involve more than the superficial layers of the skin should not be routinely treated
- repeated general skin cleansing of large body areas should not be done except when advised by a health care provider
-
Directions
- use with care in premature infants or infants under 2 months of age. These products may cause irritation or chemical burns.
Surgical hand scrub
- wet hands and forearms with water
- scrub for 3 minutes with about 5 mL of product and a wet brush, paying close attention to the nails, cuticles, and interdigital spaces
- a separate nail cleaner may be used
- rinse thoroughly
- wash for an additional 3 minutes with 5 mL of product and rinse under running water
- dry thoroughly
- Other information
- Inactive ingredients
- Questions or comments?
- PRINCIPAL DISPLAY PANEL - 946 mL Bottle Label
-
INGREDIENTS AND APPEARANCE
BACTOSHIELD CHG
chlorhexidine gluconate solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11084-039 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORHEXIDINE GLUCONATE (UNII: MOR84MUD8E) (CHLORHEXIDINE - UNII:R4KO0DY52L) CHLORHEXIDINE GLUCONATE 40 mg in 1 mL Inactive Ingredients Ingredient Name Strength COCO DIETHANOLAMIDE (UNII: 92005F972D) GLUCONOLACTONE (UNII: WQ29KQ9POT) HYDROXYETHYL CELLULOSE (5000 MPA.S AT 1%) (UNII: X70SE62ZAR) ISOPROPYL ALCOHOL (UNII: ND2M416302) LAURAMINE OXIDE (UNII: 4F6FC4MI8W) PEG-75 LANOLIN (UNII: 09179OX7TB) WATER (UNII: 059QF0KO0R) TRIDECYL ALCOHOL (UNII: 8I9428H868) Product Characteristics Color YELLOW Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11084-039-03 118 mL in 1 BOTTLE; Type 0: Not a Combination Product 12/01/2022 2 NDC:11084-039-24 946 mL in 1 BOTTLE; Type 0: Not a Combination Product 12/01/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA019125 12/01/2022 Labeler - SC Johnson Professional USA, Inc. (607378015) Establishment Name Address ID/FEI Business Operations Xttrium Laboratories, Inc. 007470579 MANUFACTURE(11084-039)