Label: RUGBY PAIN RELIEVING ANALGESIC- menthol, unspecified form and methyl salicylate cream
- NDC Code(s): 0536-1101-45
- Packager: Rugby Laboratories
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 13, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Use
-
Warnings
For external use only
Do not use
- ♦
- immediately after shower or bath
- ♦
- with a heating pad
- ♦
- on wounds, damaged, broken or irritated skin
When using this product
- ♦
- use only as directed
- ♦
- do not swallow
- ♦
- do not bandage tightly
- ♦
- avoid contact with eyes and mucous membranes
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
- SPL UNCLASSIFIED SECTION
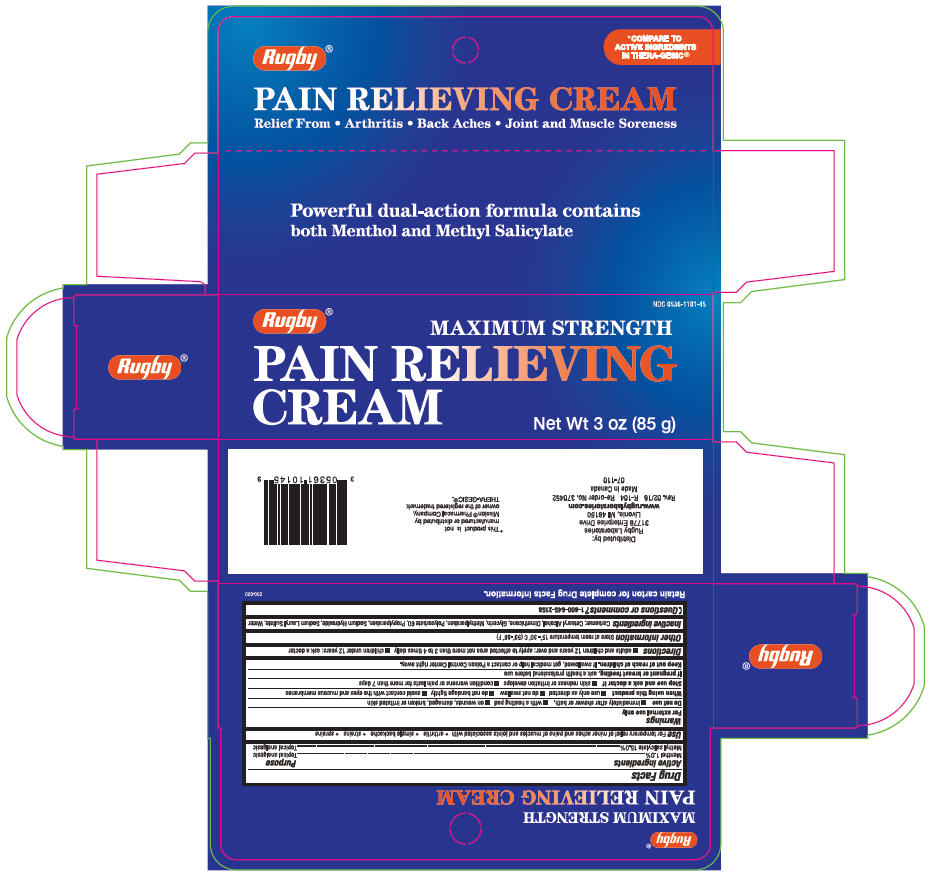
- PRINCIPAL DISPLAY PANEL - 85 g Tube Carton
-
INGREDIENTS AND APPEARANCE
RUGBY PAIN RELIEVING ANALGESIC
menthol, unspecified form and methyl salicylate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0536-1101 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) (MENTHOL, UNSPECIFIED FORM - UNII:L7T10EIP3A) MENTHOL, UNSPECIFIED FORM 10 mg in 1 g Methyl Salicylate (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) Methyl Salicylate 150 mg in 1 g Inactive Ingredients Ingredient Name Strength CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) Cetostearyl Alcohol (UNII: 2DMT128M1S) Dimethicone (UNII: 92RU3N3Y1O) Glycerin (UNII: PDC6A3C0OX) Methylparaben (UNII: A2I8C7HI9T) Polysorbate 60 (UNII: CAL22UVI4M) Propylparaben (UNII: Z8IX2SC1OH) Sodium Hydroxide (UNII: 55X04QC32I) Sodium Lauryl Sulfate (UNII: 368GB5141J) Water (UNII: 059QF0KO0R) Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0536-1101-45 1 in 1 CARTON 05/11/2016 1 85 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M017 05/11/2016 Labeler - Rugby Laboratories (079246066) Registrant - Garcoa, Inc. (103039178) Establishment Name Address ID/FEI Business Operations Garcoa, Inc. 036464697 MANUFACTURE(0536-1101)