Label: LANSOPRAZOLE tablet, orally disintegrating, delayed release
- NDC Code(s): 30142-192-55
- Packager: Kroger Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated October 24, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each tablet)
- Purpose
- Use
-
Warnings
Allergy alert: do not use if you are allergic to lansoprazole
Do not use
- •
- if you have trouble or pain swallowing food, vomiting with blood, or bloody or black stools. These may be signs of a serious condition. See your doctor.
Ask a doctor before use if you have
- •
- liver disease
- •
- had heartburn over 3 months. This may be a sign of a more serious condition.
- •
- heartburn with lightheadedness, sweating or dizziness
- •
- chest pain or shoulder pain with shortness of breath; sweating; pain spreading to arms, neck or shoulders; or lightheadedness
- •
- frequent chest pain
- •
- frequent wheezing, particularly with heartburn
- •
- unexplained weight loss
- •
- nausea or vomiting
- •
- stomach pain
Ask a doctor or pharmacist before use if you are taking
taking a prescription drug. Acid reducers may interact with certain prescription drugs.
-
Directions
- •
- adults 18 years of age and older
- •
- this product is to be used once a day (every 24 hours), every day for 14 days
- •
- it may take 1 to 4 days for full effect, although some people get complete relief of symptoms within 24 hours
14-Day Course of Treatment
- •
- take 1 tablet before eating in the morning
- •
- do not crush or chew tablets
- •
- place the tablet on tongue; tablet disintegrates, with or without water. The tablets can also be swallowed whole with water.
- •
- take every day for 14 days
- •
- do not take more than 1 tablet a day
- •
- do not use for more than 14 days unless directed by your doctor
- •
- do not take this medicine with alcohol
Repeated 14-Day Course (if needed)
- •
- you may repeat a 14-day course every 4 months
- •
- do not take for more than 14 days or more often than every 4 months unless directed by a doctor
- •
- children under 18 years of age: ask a doctor before use. Heartburn in children may sometimes be caused by a serious condition.
- Other information
-
Inactive ingredients
ascorbic acid, cetyl alcohol, colloidal silicon dioxide, copovidone, crospovidone, flavor, hypromellose, hypromellose phthalate, maize maltodextrin, maltitol, mannitol, meglumine, microcrystalline cellulose, polysorbate 80, propylene glycol, silicon dioxide, sodium stearyl fumarate, sorbitol, sucralose, sugar spheres, talc, titanium dioxide, triethyl citrate
- Questions or comments?
-
Consumer Information
Treats Frequent Heartburn
Lansoprazole
Delayed Release
Orally Disintegrating Tablets 15 mg
acid reducer
• May take 1 to 4 days for full effect
Please read the entire package insert before taking
Lansoprazole Delayed Release Orally Disintegrating Tablets.
Save for future reference.
How Lansoprazole delayed release orally disintegrating tablets Treats Your Frequent Heartburn
Lansoprazole delayed release orally disintegrating tablets stops acid production at the source - the pumps that release acid into the stomach. Lansoprazole delayed release orally disintegrating tablet is taken once a day (every 24 hours), every day for 14 days.
What You Can Expect When Taking Lansoprazole delayed release orally disintegrating tablets
Frequent heartburn can occur anytime during the 24-hour period (day or night). Take Lansoprazole delayed release orally disintegrating tablets in the morning before eating. Lansoprazole is clinically proven to treat frequent heartburn. Although some people get complete relief of symptoms within 24 hours, it may take 1 to 4 days for full effect. Make sure you take Lansoprazole delayed release orally disintegrating tablets every day for 14 days to treat your frequent heartburn.
Who Should Take Lansoprazole delayed release orally disintegrating tablets
Adults (18 years and older) with frequent heartburn - when you have heartburn 2 or more days a week.
Who Should NOT Take Lansoprazole delayed release orally disintegrating tablets
People who have one episode of heartburn a week or less, or who want immediate relief of heartburn.
How to Take Lansoprazole delayed release orally disintegrating tablets
14-DAY Course of Treatment
- •
- Take 1 tablet before eating in the morning.
- •
- Do not crush or chew tablets.
- •
- Place the tablet on tongue; tablet disintegrates, with or without water. The tablets can also be swallowed whole with water.
- •
- Take every day for 14 days.
- •
- Do not take more than 1 tablet a day.
- •
- Do not use for more than 14 days unless directed by your doctor.
- •
- Do not take this medicine with alcohol.
When to Take Lansoprazole delayed release orally disintegrating tablets Again
You may repeat a 14-day course of therapy every 4 months.
When to Talk to Your Doctor
Do not take for more than 14 days or more often than every 4 months unless directed by a doctor.
Warnings and When to Ask Your Doctor
Allergy alert: Do not use if you are allergic to lansoprazole
Do not use
- •
- if you have trouble or pain swallowing food, vomiting with blood, or bloody or black stools. These may be signs of a serious condition. See your doctor.
Ask a doctor before use if you have
- •
- liver disease
- •
- had heartburn over 3 months. This may be a sign of a more serious condition.
- •
- heartburn with lightheadedness, sweating or dizziness
- •
- chest pain or shoulder pain with shortness of breath; sweating; pain spreading to arms, neck or shoulders; or lightheadedness
- •
- frequent chest pain
- •
- frequent wheezing, particularly with heartburn
- •
- unexplained weight loss
- •
- nausea or vomiting
- •
- stomach pain
Ask a doctor or pharmacist before use if you are
taking a prescription drug. Acid reducers may interact with certain prescription drugs.
Stop use and ask a doctor if
- •
- your heartburn continues or worsens
- •
- you need to take this product for more than 14 days
- •
- you need to take more than 1 course of treatment every 4 months
- •
- you get diarrhea
- •
- you develop a rash or joint pain
If pregnant or breast-feeding, ask a health care professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away (1-800-222-1222).
Tips for Managing Heartburn
- •
- Avoid foods or drinks that are more likely to cause heartburn, such as rich, spicy, fatty and fried foods, chocolate, caffeine, alcohol and even some acidic fruits and vegetables.
- •
- Eat slowly and do not eat big meals.
- •
- Do not eat late at night or just before bedtime.
- •
- Do not lie flat or bend over soon after eating.
- •
- Raise the head of your bed.
- •
- Wear loose-fitting clothing around your stomach.
- •
- If you are overweight, lose weight.
- •
- If you smoke, quit smoking.
Clinical studies prove that lansoprazole effectively treats frequent heartburn
In three clinical studies, lansoprazole was shown to be significantly better than placebo in treating frequent heartburn.
How Lansoprazole delayed release orally disintegrating tablets is Sold
This medicine is available in 14 tablet, 28 tablet and 42 tablet sizes. These sizes contain one, two and three 14-day courses of treatment, respectively. Do not use for more than 14 days in a row unless directed by your doctor. For the 28 count (two 14-day courses) and the 42 count (three 14-day courses), you may repeat a 14-day course every 4 months.
For Questions or Comments About Lansoprazole delayed release orally disintegrating tablets
Call 1-800-719-9260
weekdays 7:30 AM to 5:00 PM EST
Made in Israel
Manufactured by:
Dexcel Pharma Technologies Ltd
10 Hakidma Street, Yokneam 2069200, Israel
:8U100 00 J3
1249460078-B
-
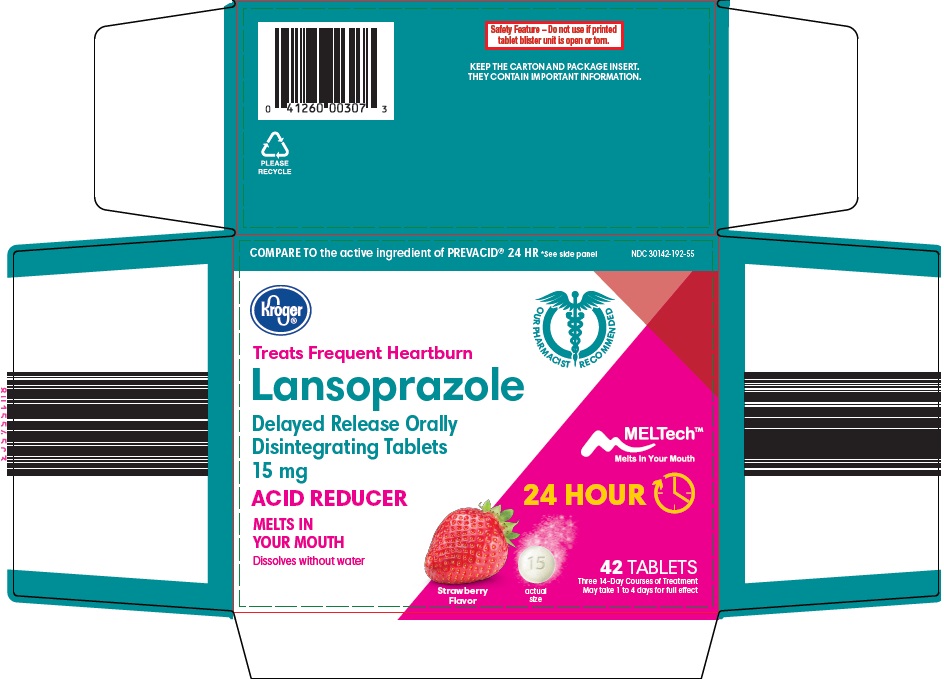
Package/Label Principal Display Panel
COMPARE TO the active ingredient of PREVACID® 24 HR See side panel
OUR PHARMACIST RECOMMENDED
Treats Frequent Heartburn
Lansoprazole Delayed Release Orally Disintegrating Tablets 15 mg
MELTech™
Melts In Your Mouth
ACID REDUCER
24 HOUR
MELTS IN YOUR MOUTH
Dissolves without water
Strawberry Flavor
actual size
42 TABLETS
Three 14-Day Courses of Treatment
May take 1 to 4 days for full effect
-
INGREDIENTS AND APPEARANCE
LANSOPRAZOLE
lansoprazole tablet, orally disintegrating, delayed releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:30142-192 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LANSOPRAZOLE (UNII: 0K5C5T2QPG) (LANSOPRAZOLE - UNII:0K5C5T2QPG) LANSOPRAZOLE 15 mg Inactive Ingredients Ingredient Name Strength ASCORBIC ACID (UNII: PQ6CK8PD0R) CETYL ALCOHOL (UNII: 936JST6JCN) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) COPOVIDONE K25-31 (UNII: D9C330MD8B) CROSPOVIDONE (15 MPA.S AT 5%) (UNII: 68401960MK) HYPROMELLOSES (UNII: 3NXW29V3WO) MALTITOL (UNII: D65DG142WK) MANNITOL (UNII: 3OWL53L36A) MEGLUMINE (UNII: 6HG8UB2MUY) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYSORBATE 80 (UNII: 6OZP39ZG8H) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SODIUM STEARYL FUMARATE (UNII: 7CV7WJK4UI) SORBITOL (UNII: 506T60A25R) SUCRALOSE (UNII: 96K6UQ3ZD4) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) Product Characteristics Color WHITE (off white mottled) Score no score Shape ROUND Size 9mm Flavor Imprint Code 15 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:30142-192-55 3 in 1 CARTON 04/05/2019 1 14 in 1 CARTON 1 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA208025 04/05/2019 Labeler - Kroger Company (006999528)