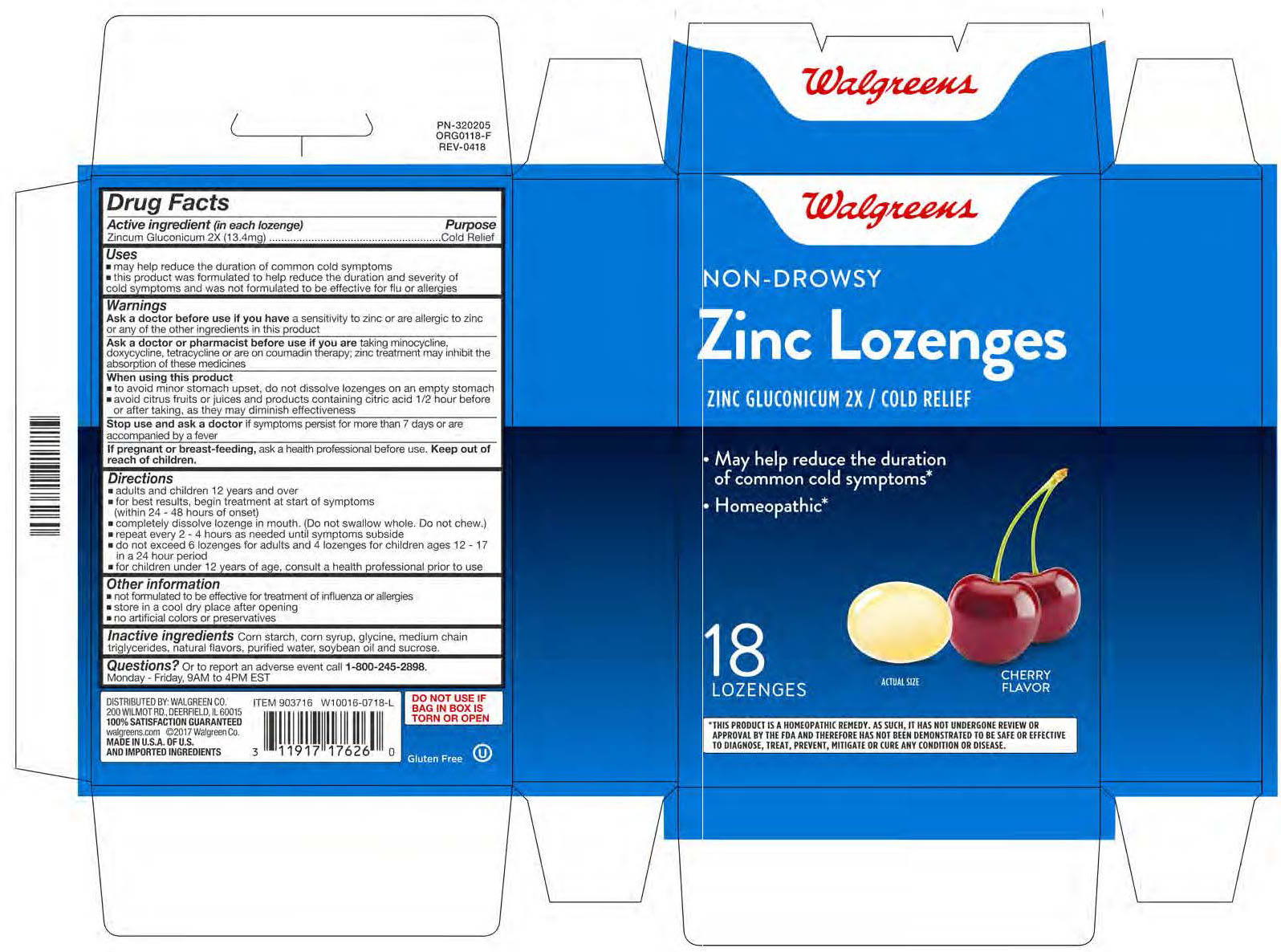
Label: COLD RELIEF- zinc gluconate lozenge
-
Contains inactivated NDC Code(s)
NDC Code(s): 0363-1090-18 - Packager: Walgreen Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated December 28, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- ASK DOCTOR/PHARMACIST
- WHEN USING
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions
adults and children 12 years and over
for best results, begin treatment at start of symptoms (within 24 - 48 hours of onset)
completely dissolve lozenges in mouth. (Do not swallow whole. Do not chew.)
repeat every 2 - 4 hours as needed until symptoms subside
do not exceed 6 lozenges for adults and 4 lozenges for children ages 12 - 17 in a 24 hour period
for children under 12 years of age, consult a health professional prior to use
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
COLD RELIEF
zinc gluconate lozengeProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0363-1090 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC GLUCONATE (UNII: U6WSN5SQ1Z) (ZINC CATION - UNII:13S1S8SF37) ZINC GLUCONATE 2 [hp_X] Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) Product Characteristics Color white (Semi-Translucent) Score no score Shape OVAL Size 16mm Flavor CHERRY Imprint Code B Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0363-1090-18 18 in 1 CARTON; Type 0: Not a Combination Product 06/11/2015 
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 06/11/2015 Labeler - Walgreen Company (008965063) Registrant - Bestco Inc. (002149136) Establishment Name Address ID/FEI Business Operations Bestco Inc. 002149136 manufacture(0363-1090)