Label: DARZALEX FASPRO (daratumumab and hyaluronidase-fihj- human recombinant injection
- NDC Code(s): 57894-503-01
- Packager: Janssen Biotech, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated July 5, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use DARZALEX FASPRO safely and effectively. See full prescribing information for DARZALEX FASPRO.
DARZALEX FASPRO ® (daratumumab and hyaluronidase-fihj) injection, for subcutaneous use
Initial U.S. Approval: 2020INDICATIONS AND USAGE
DARZALEX FASPRO is a combination of daratumumab, a CD38-directed cytolytic antibody, and hyaluronidase, an endoglycosidase, indicated for the treatment of adult patients with:
- multiple myeloma in combination with bortezomib, melphalan and prednisone in newly diagnosed patients who are ineligible for autologous stem cell transplant
- multiple myeloma in combination with lenalidomide and dexamethasone in newly diagnosed patients who are ineligible for autologous stem cell transplant and in patients with relapsed or refractory multiple myeloma who have received at least one prior therapy
- multiple myeloma in combination with bortezomib, thalidomide, and dexamethasone in newly diagnosed patients who are eligible for autologous stem cell transplant
- multiple myeloma in combination with bortezomib and dexamethasone in patients who have received at least one prior therapy
- multiple myeloma in combination with pomalidomide and dexamethasone in patients who have received at least one prior line of therapy including lenalidomide and a proteasome inhibitor
- multiple myeloma in combination with carfilzomib and dexamethasone in patients with relapsed or refractory multiple myeloma who have received one to three prior lines of therapy
- multiple myeloma as monotherapy, in patients who have received at least three prior lines of therapy including a proteasome inhibitor (PI) and an immunomodulatory agent or who are double-refractory to a PI and an immunomodulatory agent
- light chain (AL) amyloidosis in combination with bortezomib, cyclophosphamide and dexamethasone in newly diagnosed patients. This indication is approved under accelerated approval based on response rate. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial(s) ( 1.2)
Limitations of Use:
- DARZALEX FASPRO is not indicated and is not recommended for the treatment of patients with light chain (AL) amyloidosis who have NYHA Class IIIB or Class IV cardiac disease or Mayo Stage IIIB outside of controlled clinical trials ( 1.2)
DOSAGE AND ADMINISTRATION
For subcutaneous use only.
- Pre-medicate with a corticosteroid, acetaminophen and a histamine-1 receptor antagonist. ( 2.5)
- The recommended dosage of DARZALEX FASPRO is (1,800 mg daratumumab and 30,000 units hyaluronidase) administered subcutaneously into the abdomen over approximately 3 to 5 minutes according to recommended schedule. ( 2.2, 2.3)
- Administer post-medications as recommended. ( 2.5)
DOSAGE FORMS AND STRENGTHS
- Injection: 1,800 mg daratumumab and 30,000 units hyaluronidase per 15 mL (120 mg and 2,000 units/mL) solution in a single-dose vial ( 3)
CONTRAINDICATIONS
Patients with a history of severe hypersensitivity to daratumumab, hyaluronidase or any of the components of the formulation. ( 4)
WARNINGS AND PRECAUTIONS
- Hypersensitivity and Other Administration Reactions: Permanently discontinue DARZALEX FASPRO for life-threatening reactions. ( 5.1)
- Cardiac Toxicity in Patients with Light Chain (AL) Amyloidosis: Monitor patients with cardiac involvement more frequently for cardiac adverse reactions and administer supportive care as appropriate. ( 5.2)
- Neutropenia: Monitor complete blood cell counts periodically during treatment. Monitor patients with neutropenia for signs of infection. Consider withholding DARZALEX FASPRO to allow recovery of neutrophils. ( 5.3)
- Thrombocytopenia: Monitor complete blood cell counts periodically during treatment. Consider withholding DARZALEX FASPRO to allow recovery of platelets. ( 5.4)
- Embryo-Fetal Toxicity: Can cause fetal harm. Advise pregnant women of the potential risk to a fetus and advise females of reproductive potential to use effective contraception. ( 5.5, 8.1, 8.3)
- Interference with cross-matching and red blood cell antibody screening: Type and screen patients prior to starting treatment. Inform blood banks that a patient has received DARZALEX FASPRO. ( 5.6, 7.1)
ADVERSE REACTIONS
- The most common adverse reaction (≥20%) in patients with multiple myeloma who received DARZALEX FASPRO monotherapy is upper respiratory tract infection. ( 6.1)
- The most common adverse reactions (≥20%) in patients with multiple myeloma who received DARZALEX FASPRO-VMP are upper respiratory tract infection, constipation, nausea, fatigue, pyrexia, peripheral sensory neuropathy, diarrhea, cough, insomnia, vomiting, and back pain. ( 6.1)
- The most common adverse reactions (≥20%) in patients with multiple myeloma who received DARZALEX FASPRO-Rd are fatigue, diarrhea, upper respiratory tract infection, muscle spasms, constipation, pyrexia, pneumonia, and dyspnea. ( 6.1)
- The most common adverse reactions (≥20%) in patients with multiple myeloma who received DARZALEX FASPRO-Pd are fatigue, pneumonia, upper respiratory tract infection, and diarrhea. ( 6.1)
- The most common adverse reactions (≥20%) in patients with multiple myeloma who received DARZALEX FASPRO-Kd are upper respiratory tract infection, fatigue, insomnia, hypertension, diarrhea, cough, dyspnea, headache, pyrexia, nausea, and edema peripheral. ( 6.1)
- The most common adverse reactions (≥20%) in patients with light chain (AL) amyloidosis are upper respiratory tract infection, diarrhea, peripheral edema, constipation, fatigue, peripheral sensory neuropathy, nausea, insomnia, dyspnea, and cough. ( 6.1)
- The most common (≥40%) hematology laboratory abnormalities with DARZALEX FASPRO are decreased leukocytes, decreased lymphocytes, decreased neutrophils, decreased platelets, and decreased hemoglobin. ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Janssen Biotech, Inc. at 1-800-526-7736 (1-800-JANSSEN) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 12/2022
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Multiple Myeloma
1.2 Light Chain Amyloidosis
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosing Information
2.2 Recommended Dosage for Multiple Myeloma
2.3 Recommended Dosage for Light Chain Amyloidosis
2.4 Administration
2.5 Recommended Concomitant Medications
2.6 Dosage Modifications for Adverse Reactions
2.7 Preparation and Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity and Other Administration Reactions
5.2 Cardiac Toxicity in Patients with Light Chain (AL) Amyloidosis
5.3 Neutropenia
5.4 Thrombocytopenia
5.5 Embryo-Fetal Toxicity
5.6 Interference with Serological Testing
5.7 Interference with Determination of Complete Response
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Immunogenicity
6.3 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Effects of Daratumumab on Laboratory Tests
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Newly Diagnosed Multiple Myeloma
14.2 Relapsed/Refractory Multiple Myeloma
14.3 Light Chain Amyloidosis
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Multiple Myeloma
DARZALEX FASPRO is indicated for the treatment of adult patients with multiple myeloma:
- in combination with bortezomib, melphalan and prednisone in newly diagnosed patients who are ineligible for autologous stem cell transplant.
- in combination with lenalidomide and dexamethasone in newly diagnosed patients who are ineligible for autologous stem cell transplant and in patients with relapsed or refractory multiple myeloma who have received at least one prior therapy.
- in combination with bortezomib, thalidomide, and dexamethasone in newly diagnosed patients who are eligible for autologous stem cell transplant.
- in combination with bortezomib and dexamethasone in patients who have received at least one prior therapy.
- in combination with pomalidomide and dexamethasone in patients who have received at least one prior line of therapy including lenalidomide and a proteasome inhibitor.
- in combination with carfilzomib and dexamethasone in patients with relapsed or refractory multiple myeloma who have received one to three prior lines of therapy.
- as monotherapy, in patients who have received at least three prior lines of therapy including a proteasome inhibitor (PI) and an immunomodulatory agent or who are double-refractory to a PI and an immunomodulatory agent.
1.2 Light Chain Amyloidosis
DARZALEX FASPRO in combination with bortezomib, cyclophosphamide and dexamethasone is indicated for the treatment of adult patients with newly diagnosed light chain (AL) amyloidosis.
This indication is approved under accelerated approval based on response rate [see Clinical Studies (14.3)] . Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial(s).
Limitations of Use
DARZALEX FASPRO is not indicated and is not recommended for the treatment of patients with light chain (AL) amyloidosis who have NYHA Class IIIB or Class IV cardiac disease or Mayo Stage IIIB outside of controlled clinical trials [see Warnings and Precautions (5.2)] .
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosing Information
- DARZALEX FASPRO is for subcutaneous use only.
- Administer medications before and after administration of DARZALEX FASPRO to minimize administration-related reactions [see Dosage and Administration (2.5)] .
- Type and screen patients prior to starting DARZALEX FASPRO.
2.2 Recommended Dosage for Multiple Myeloma
The recommended dose of DARZALEX FASPRO is 1,800 mg/30,000 units (1,800 mg daratumumab and 30,000 units hyaluronidase) administered subcutaneously over approximately 3–5 minutes. Tables 1, 2, 3, and 4 provide the recommended dosing schedule when DARZALEX FASPRO is administered as monotherapy or as part of a combination therapy.
Monotherapy and In Combination with Lenalidomide and Dexamethasone (DARZALEX FASPRO-Rd), Pomalidomide and Dexamethasone (DARZALEX FASPRO-Pd) or Carfilzomib and Dexamethasone (DARZALEX FASPRO-Kd)
Use the dosing schedule provided in Table 1 when DARZALEX FASPRO is administered:
- in combination with lenalidomide and dexamethasone (4-week cycle) OR
- in combination with pomalidomide and dexamethasone (4-week cycle) OR
- in combination with carfilzomib and dexamethasone (4-week cycle) OR
- as monotherapy.
Table 1: DARZALEX FASPRO dosing schedule in combination with lenalidomide, pomalidomide or carfilzomib and dexamethasone (4-week cycle) and for monotherapy Weeks Schedule Weeks 1 to 8 weekly (total of 8 doses) Weeks 9 to 24 * every two weeks (total of 8 doses) Week 25 onwards until disease progression † every four weeks When DARZALEX FASPRO is administered as part of a combination therapy, see Clinical Studies (14.2) and the prescribing information for dosage recommendations for the other drugs.
In Combination with Bortezomib, Melphalan and Prednisone (DARZALEX FASPRO-VMP)
Use the dosing schedule provided in Table 2 when DARZALEX FASPRO is administered in combination with bortezomib, melphalan and prednisone (6-week cycle).
Table 2: DARZALEX FASPRO dosing schedule in combination with bortezomib, melphalan and prednisone (6-week cycle) Weeks Schedule Weeks 1 to 6 weekly (total of 6 doses) Weeks 7 to 54 * every three weeks (total of 16 doses) Week 55 onwards until disease progression † every four weeks When DARZALEX FASPRO is administered as part of a combination therapy, see Clinical Studies (14.1) and the prescribing information for dosage recommendations for the other drugs.
In Combination with Bortezomib, Thalidomide, and Dexamethasone (DARZALEX FASPRO-VTd)
Use the dosing schedule in Table 3 when DARZALEX FASPRO is administered in combination with bortezomib, thalidomide, and dexamethasone (4-week cycle).
Table 3: DARZALEX FASPRO dosing schedule in combination with bortezomib, thalidomide and dexamethasone (4-week cycle) Treatment phase Weeks Schedule Induction Weeks 1 to 8 weekly (total of 8 doses) Weeks 9 to 16 * every two weeks (total of 4 doses) Stop for high dose chemotherapy and ASCT Consolidation Weeks 1 to 8 † every two weeks (total of 4 doses) When DARZALEX FASPRO is administered as part of a combination therapy, see the prescribing information for dosage recommendations for the other drugs.
In Combination with Bortezomib and Dexamethasone (DARZALEX FASPRO-Vd)
Use the dosing schedule in Table 4 when DARZALEX FASPRO is administered in combination with bortezomib and dexamethasone (3-week cycle).
Table 4: DARZALEX FASPRO dosing schedule in combination with bortezomib and dexamethasone (3-week cycle) Weeks Schedule Weeks 1 to 9 weekly (total of 9 doses) Weeks 10 to 24 * every three weeks (total of 5 doses) Week 25 onwards until disease progression † every four weeks When DARZALEX FASPRO is administered as part of a combination therapy, see the prescribing information for dosage recommendations for the other drugs.
2.3 Recommended Dosage for Light Chain Amyloidosis
In Combination with Bortezomib, Cyclophosphamide and Dexamethasone (DARZALEX FASPRO-VCd)
Use the dosing schedule provided in Table 5 when DARZALEX FASPRO is administered in combination with bortezomib, cyclophosphamide and dexamethasone (4-week cycle).
Table 5: DARZALEX FASPRO dosing schedule in combination with bortezomib, cyclophosphamide and dexamethasone (4-week cycle) Weeks Schedule Weeks 1 to 8 weekly (total of 8 doses) Weeks 9 to 24 * every two weeks (total of 8 doses) Week 25 onwards until disease progression or a maximum of 2 years † every four weeks When DARZALEX FASPRO is administered as part of a combination therapy, see Clinical Studies (14.2) and the prescribing information for dosage recommendations for the other drugs.
2.4 Administration
If a dose of DARZALEX FASPRO is missed, administer the dose as soon as possible and adjust the dosing schedule to maintain the dosing interval.
2.5 Recommended Concomitant Medications
Pre-medication
Administer the following pre-medications 1–3 hours before each dose of DARZALEX FASPRO:
- Acetaminophen 650 to 1,000 mg orally
- Diphenhydramine 25 to 50 mg (or equivalent) orally or intravenously
- Corticosteroid (long- or intermediate-acting)
Monotherapy
Administer methylprednisolone 100 mg (or equivalent) orally or intravenously. Consider reducing the dose of methylprednisolone to 60 mg (or equivalent) following the second dose of DARZALEX FASPRO.
In Combination
Administer dexamethasone 20 mg (or equivalent) orally or intravenously prior to every DARZALEX FASPRO administration.
When dexamethasone is the background regimen-specific corticosteroid, the dexamethasone dose that is part of the background regimen will serve as pre-medication on DARZALEX FASPRO administration days [see Clinical Studies (14)] .
Do not administer background regimen-specific corticosteroids (e.g. prednisone) on DARZALEX FASPRO administration days when patients have received dexamethasone (or equivalent) as a pre-medication.
Post-medication
Administer the following post-medications:
-
Monotherapy
Administer methylprednisolone 20 mg (or an equivalent dose of an intermediate- or long-acting corticosteroid) orally for 2 days starting the day after the administration of DARZALEX FASPRO. -
In Combination
Consider administering oral methylprednisolone at a dose of less than or equal to 20 mg (or an equivalent dose of an intermediate- or long-acting corticosteroid) beginning the day after administration of DARZALEX FASPRO.
If a background regimen-specific corticosteroid (e.g. dexamethasone, prednisone) is administered the day after the administration of DARZALEX FASPRO, additional corticosteroids may not be needed [see Clinical Studies (14)] .
If the patient does not experience a major systemic administration-related reaction after the first 3 doses of DARZALEX FASPRO, consider discontinuing the administration of corticosteroids (excluding any background regimen-specific corticosteroid).
For patients with a history of chronic obstructive pulmonary disease, consider prescribing short and long-acting bronchodilators and inhaled corticosteroids. Following the first 4 doses of DARZALEX FASPRO, consider discontinuing these additional post-medications, if the patient does not experience a major systemic administration-related reaction.
Prophylaxis for Herpes Zoster Reactivation
Initiate antiviral prophylaxis to prevent herpes zoster reactivation within 1 week after starting DARZALEX FASPRO and continue for 3 months following the end of treatment [see Adverse Reactions (6.1)].
2.6 Dosage Modifications for Adverse Reactions
No dose reductions of DARZALEX FASPRO are recommended. Consider withholding DARZALEX FASPRO to allow recovery of blood cell counts in the event of myelosuppression [see Warnings and Precautions (5.3, 5.4)] .
2.7 Preparation and Administration
DARZALEX FASPRO should be administered by a healthcare provider.
To prevent medication errors, check the vial labels to ensure that the drug being prepared and administered is DARZALEX FASPRO for subcutaneous use. Do not administer DARZALEX FASPRO intravenously.
DARZALEX FASPRO is ready to use.
Preparation
- Remove the DARZALEX FASPRO vial from refrigerated storage [2°C to 8°C (36°F to 46°F)] and equilibrate to ambient temperature [15°C to 30°C (59°F to 86°F)]. Store the unpunctured vial at ambient temperature and ambient light for a maximum of 24 hours. Keep out of direct sunlight. Do not shake.
- Withdraw 15 mL from the vial into a syringe.
- DARZALEX FASPRO is compatible with polypropylene or polyethylene syringe material; polypropylene, polyethylene, or polyvinyl chloride (PVC) subcutaneous infusion sets; and stainless steel transfer and injection needles. Use the product immediately.
- After the solution of DARZALEX FASPRO is withdrawn into the syringe, replace the transfer needle with a syringe closing cap. Label the syringe appropriately to include the route of administration per institutional standards. Label the syringe with the peel-off label.
- To avoid needle clogging, attach the hypodermic injection needle or subcutaneous infusion set to the syringe immediately prior to injection.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use if opaque particles, discoloration or other foreign particles are present.
Storage
- If the syringe containing DARZALEX FASPRO is not used immediately, store refrigerated at 2°C to 8°C (36°F to 46°F) for up to 24 hours and/or at room temperature at 15°C to 25°C (59°F to 77°F) for up to 12 hours under ambient light.
- Discard if storage time exceeds these limits.
- If stored in the refrigerator, allow the solution to come to room temperature before administration.
Administration
- Inject 15 mL of DARZALEX FASPRO into the subcutaneous tissue of the abdomen approximately 3 inches [7.5 cm] to the right or left of the navel over approximately 3–5 minutes. No data are available on performing the injection at other sites of the body.
- Rotate injection sites for successive injections.
- Never inject DARZALEX FASPRO into areas where the skin is red, bruised, tender, hard or areas where there are scars.
- Pause or slow down delivery rate if the patient experiences pain. In the event pain is not alleviated by pausing or slowing down delivery rate, a second injection site may be chosen on the opposite side of the abdomen to deliver the remainder of the dose.
- During treatment with DARZALEX FASPRO, do not administer other medications for subcutaneous use at the same site as DARZALEX FASPRO.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
DARZALEX FASPRO is contraindicated in patients with a history of severe hypersensitivity to daratumumab, hyaluronidase or any of the components of the formulation [see Warnings and Precautions (5.1) and Adverse Reactions (6.3)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity and Other Administration Reactions
Both systemic administration-related reactions, including severe or life-threatening reactions, and local injection-site reactions can occur with DARZALEX FASPRO. Fatal reactions have been reported with daratumumab-containing products, including DARZALEX FASPRO [see Adverse Reactions (6.3)] .
Systemic Reactions
In a pooled safety population of 898 patients with multiple myeloma (N=705) or light chain (AL) amyloidosis (N=193) who received DARZALEX FASPRO as monotherapy or as part of a combination therapy, 9% of patients experienced a systemic administration-related reaction (Grade 2: 3.2%, Grade 3: 1%). Systemic administration-related reactions occurred in 8% of patients with the first injection, 0.3% with the second injection, and cumulatively 1% with subsequent injections. The median time to onset was 3.2 hours (range: 4 minutes to 3.5 days). Of the 140 systemic administration-related reactions that occurred in 77 patients, 121 (86%) occurred on the day of DARZALEX FASPRO administration. Delayed systemic administration-related reactions have occurred in 1% of the patients.
Severe reactions include hypoxia, dyspnea, hypertension, and tachycardia, and ocular adverse reactions, including choroidal effusion, acute myopia, and acute angle closure glaucoma. Other signs and symptoms of systemic administration-related reactions may include respiratory symptoms, such as bronchospasm, nasal congestion, cough, throat irritation, allergic rhinitis, and wheezing, as well as anaphylactic reaction, pyrexia, chest pain, pruritus, chills, vomiting, nausea, hypotension, and blurred vision.
Pre-medicate patients with histamine-1 receptor antagonist, acetaminophen and corticosteroids [see Dosage and Administration (2.5)] . Monitor patients for systemic administration-related reactions, especially following the first and second injections. For anaphylactic reaction or life-threatening (Grade 4) administration-related reactions, immediately and permanently discontinue DARZALEX FASPRO. Consider administering corticosteroids and other medications after the administration of DARZALEX FASPRO depending on dosing regimen and medical history to minimize the risk of delayed (defined as occurring the day after administration) systemic administration-related reactions [see Dosage and Administration (2.5)] .
Ocular adverse reactions, including acute myopia and narrowing of the anterior chamber angle due to ciliochoroidal effusions with potential for increased intraocular pressure or glaucoma, have occurred with daratumumab-containing products. If ocular symptoms occur, interrupt DARZALEX FASPRO and seek immediate ophthalmologic evaluation prior to restarting DARZALEX FASPRO.
Local Reactions
In this pooled safety population, injection-site reactions occurred in 8% of patients, including Grade 2 reactions in 0.7%. The most frequent (>1%) injection-site reaction was injection site erythema. These local reactions occurred a median of 5 minutes (range: 0 minutes to 6.5 days) after starting administration of DARZALEX FASPRO. Monitor for local reactions and consider symptomatic management.
5.2 Cardiac Toxicity in Patients with Light Chain (AL) Amyloidosis
Serious or fatal cardiac adverse reactions occurred in patients with light chain (AL) amyloidosis who received DARZALEX FASPRO in combination with bortezomib, cyclophosphamide and dexamethasone [see Adverse Reactions (6.1)] . Serious cardiac disorders occurred in 16% and fatal cardiac disorders occurred in 10% of patients. Patients with NYHA Class IIIA or Mayo Stage IIIA disease may be at greater risk. Patients with NYHA Class IIIB or IV disease were not studied.
Monitor patients with cardiac involvement of light chain (AL) amyloidosis more frequently for cardiac adverse reactions and administer supportive care as appropriate.
5.3 Neutropenia
Daratumumab may increase neutropenia induced by background therapy [see Adverse Reactions (6.1)] .
Monitor complete blood cell counts periodically during treatment according to manufacturer's prescribing information for background therapies. Monitor patients with neutropenia for signs of infection. Consider withholding DARZALEX FASPRO until recovery of neutrophils. In lower body weight patients receiving DARZALEX FASPRO, higher rates of Grade 3–4 neutropenia were observed.
5.4 Thrombocytopenia
Daratumumab may increase thrombocytopenia induced by background therapy [see Adverse Reactions (6.1)] .
Monitor complete blood cell counts periodically during treatment according to manufacturer's prescribing information for background therapies. Consider withholding DARZALEX FASPRO until recovery of platelets.
5.5 Embryo-Fetal Toxicity
Based on the mechanism of action, DARZALEX FASPRO can cause fetal harm when administered to a pregnant woman. DARZALEX FASPRO may cause depletion of fetal immune cells and decreased bone density. Advise pregnant women of the potential risk to a fetus. Advise females with reproductive potential to use effective contraception during treatment with DARZALEX FASPRO and for 3 months after the last dose [see Use in Specific Populations (8.1, 8.3)] .
The combination of DARZALEX FASPRO with lenalidomide, thalidomide or pomalidomide is contraindicated in pregnant women, because lenalidomide, thalidomide or pomalidomide may cause birth defects and death of the unborn child. Refer to the lenalidomide, thalidomide or pomalidomide prescribing information on use during pregnancy.
5.6 Interference with Serological Testing
Daratumumab binds to CD38 on red blood cells (RBCs) and results in a positive Indirect Antiglobulin Test (Indirect Coombs test). Daratumumab-mediated positive indirect antiglobulin test may persist for up to 6 months after the last daratumumab administration. Daratumumab bound to RBCs masks detection of antibodies to minor antigens in the patient's serum [see References (15)] . The determination of a patient's ABO and Rh blood type are not impacted [see Drug Interactions (7.1)] .
Notify blood transfusion centers of this interference with serological testing and inform blood banks that a patient has received DARZALEX FASPRO. Type and screen patients prior to starting DARZALEX FASPRO [see Dosage and Administration (2.1)] .
5.7 Interference with Determination of Complete Response
Daratumumab is a human IgG kappa monoclonal antibody that can be detected on both the serum protein electrophoresis (SPE) and immunofixation (IFE) assays used for the clinical monitoring of endogenous M-protein [see Drug Interactions (7.1)] . This interference can impact the determination of complete response and of disease progression in some DARZALEX FASPRO-treated patients with IgG kappa myeloma protein.
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Hypersensitivity and Other Administration Reactions [see Warnings and Precautions (5.1)] .
- Cardiac Toxicity in Patients with Light Chain (AL) Amyloidosis [see Warnings and Precautions (5.2)] .
- Neutropenia [see Warnings and Precautions (5.3)] .
- Thrombocytopenia [see Warnings and Precautions (5.4)] .
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Newly Diagnosed Multiple Myeloma
In Combination with Bortezomib, Melphalan and Prednisone
The safety of DARZALEX FASPRO with bortezomib, melphalan and prednisone was evaluated in a single-arm cohort of PLEIADES [see Clinical Studies (14.1)] . Patients received DARZALEX FASPRO 1,800 mg/30,000 units administered subcutaneously once weekly from weeks 1 to 6, once every 3 weeks from weeks 7 to 54 and once every 4 weeks starting with week 55 until disease progression or unacceptable toxicity (N=67) in combination with bortezomib, melphalan and prednisone. Among these patients, 93% were exposed for 6 months or longer and 19% were exposed for greater than one year.
Serious adverse reactions occurred in 39% of patients who received DARZALEX FASPRO. Serious adverse reactions in >5% of patients included pneumonia and pyrexia. Fatal adverse reactions occurred in 3% of patients.
Permanent discontinuation of DARZALEX FASPRO due to an adverse reaction occurred in 4.5% of patients. The adverse reaction resulting in permanent discontinuation of DARZALEX FASPRO in more than 1 patient was neutropenic sepsis.
Dosage interruptions (defined as dose delays or skipped doses) due to an adverse reaction occurred in 51% of patients who received DARZALEX FASPRO. Adverse reactions requiring dosage interruptions in >5% of patients included thrombocytopenia, neutropenia, anemia, and pneumonia.
The most common adverse reactions (≥20%) were upper respiratory tract infection, constipation, nausea, fatigue, pyrexia, peripheral sensory neuropathy, diarrhea, cough, insomnia, vomiting, and back pain.
Table 6 summarizes the adverse reactions in patients who received DARZALEX FASPRO in PLEIADES.
Table 6: Adverse Reactions (≥10%) in Patients Who Received DARZALEX FASPRO with Bortezomib, Melphalan and Prednisone (DARZALEX FASPRO-VMP) in PLEIADES Adverse Reaction DARZALEX FASPRO with Bortezomib, Melphalan and Prednisone
(N=67)All Grades
(%)Grades ≥3
(%)- *
- Upper respiratory tract infection includes nasopharyngitis, respiratory syncytial virus infection, respiratory tract infection, rhinitis, tonsillitis, upper respiratory tract infection, and viral pharyngitis.
- †
- Pneumonia includes lower respiratory tract infection, lung infection, pneumocystis jirovecii pneumonia, pneumonia, and pneumonia bacterial.
- ‡
- Only Grade 3 adverse reactions occurred.
- §
- Abdominal pain includes abdominal pain, and abdominal pain upper.
- ¶
- Fatigue includes asthenia, and fatigue.
- #
- Edema peripheral includes edema, edema peripheral, and peripheral swelling.
- Þ
- Cough includes cough, and productive cough.
Infections Upper respiratory tract infection * 39 0 Bronchitis 16 0 Pneumonia † 15 7 ‡ Gastrointestinal disorders Constipation 37 0 Nausea 36 0 Diarrhea 33 3 ‡ Vomiting 21 0 Abdominal pain § 13 0 General disorders and administration site conditions Fatigue ¶ 36 3 Pyrexia 34 0 Edema peripheral # 13 1 ‡ Nervous system disorders Peripheral sensory neuropathy 34 1 ‡ Dizziness 10 0 Respiratory, thoracic and mediastinal disorders Cough Þ 24 0 Psychiatric disorders Insomnia 22 3 ‡ Musculoskeletal and connective tissue disorders Back pain 21 3 ‡ Musculoskeletal chest pain 12 0 Metabolism and nutrition disorders Decreased appetite 15 1 ‡ Skin and subcutaneous tissue disorders Rash 13 0 Pruritus 12 0 Vascular disorders Hypertension 13 6 ‡ Hypotension 10 3 ‡ Clinically relevant adverse reactions in <10% of patients who received DARZALEX FASPRO with bortezomib, melphalan and prednisone included:
- General disorders and administration site conditions: infusion reaction, injection site reaction, chills
- Infections: herpes zoster, urinary tract infection, influenza, sepsis
- Musculoskeletal and connective tissue disorders: arthralgia, muscle spasms
- Nervous system disorders: headache, paresthesia
- Metabolism and nutrition disorders: hypocalcemia, hyperglycemia
- Respiratory, thoracic and mediastinal disorders: dyspnea, pulmonary edema
- Cardiac disorders: atrial fibrillation
Table 7 summarizes the laboratory abnormalities in patients who received DARZALEX FASPRO in PLEIADES.
Table 7: Select Hematology Laboratory Abnormalities Worsening from Baseline in Patients Who Received DARZALEX FASPRO with Bortezomib, Melphalan and Prednisone (DARZALEX FASPRO-VMP) in PLEIADES Laboratory Abnormality DARZALEX FASPRO with Bortezomib, Melphalan and Prednisone * All Grades
(%)Grades 3–4
(%)- *
- Denominator is based on the safety population treated with DARZALEX FASPRO-VMP (N=67).
Decreased leukocytes 96 52 Decreased lymphocytes 93 84 Decreased platelets 93 42 Decreased neutrophils 88 49 Decreased hemoglobin 48 19 Relapsed/Refractory Multiple Myeloma
In Combination with Lenalidomide and Dexamethasone
The safety of DARZALEX FASPRO with lenalidomide and dexamethasone was evaluated in a single-arm cohort of PLEIADES [see Clinical Studies (14.2)]. Patients received DARZALEX FASPRO 1,800 mg/30,000 units administered subcutaneously once weekly from weeks 1 to 8, once every 2 weeks from weeks 9 to 24 and once every 4 weeks starting with week 25 until disease progression or unacceptable toxicity (N=65) in combination with lenalidomide and dexamethasone. Among these patients, 92% were exposed for 6 months or longer and 20% were exposed for greater than one year.
Serious adverse reactions occurred in 48% of patients who received DARZALEX FASPRO. Serious adverse reactions in >5% of patients included pneumonia, influenza and diarrhea. Fatal adverse reactions occurred in 3.1% of patients.
Permanent discontinuation of DARZALEX FASPRO due to an adverse reaction occurred in 11% of patients who received DARZALEX FASPRO. Adverse reactions resulting in permanent discontinuation of DARZALEX FASPRO in more than 1 patient were pneumonia and anemia.
Dosage interruptions due to an adverse reaction occurred in 63% of patients who received DARZALEX FASPRO. Adverse reactions requiring dosage interruptions in >5% of patients included neutropenia, pneumonia, upper respiratory tract infection, influenza, dyspnea, and blood creatinine increased.
The most common adverse reactions (≥20%) were fatigue, diarrhea, upper respiratory tract infection, muscle spasms, constipation, pyrexia, pneumonia, and dyspnea.
Table 8 summarizes the adverse reactions in patients who received DARZALEX FASPRO in PLEIADES.
Table 8: Adverse Reactions (≥10%) in Patients Who Received DARZALEX FASPRO with Lenalidomide and Dexamethasone (DARZALEX FASPRO-Rd) in PLEIADES Adverse Reaction DARZALEX FASPRO with Lenalidomide and Dexamethasone
(N=65)All Grades
(%)Grades ≥3
(%)- *
- Fatigue includes asthenia, and fatigue.
- †
- Only Grade 3 adverse reactions occurred.
- ‡
- Upper respiratory tract infection includes nasopharyngitis, pharyngitis, respiratory tract infection viral, rhinitis, sinusitis, upper respiratory tract infection, and upper respiratory tract infection bacterial.
- §
- Pneumonia includes lower respiratory tract infection, lung infection, and pneumonia.
- ¶
- Bronchitis includes bronchitis, and bronchitis viral.
- #
- Dyspnea includes dyspnea, and dyspnea exertional.
- Þ
- Cough includes cough, and productive cough.
General disorders and administration site conditions Fatigue * 52 5 † Pyrexia 23 2 † Edema peripheral 18 3 † Gastrointestinal disorders Diarrhea 45 5 † Constipation 26 2 † Nausea 12 0 Vomiting 11 0 Infections Upper respiratory tract infection ‡ 43 3 † Pneumonia § 23 17 Bronchitis ¶ 14 2 † Urinary tract infection 11 0 Musculoskeletal and connective tissue disorders Muscle spasms 31 2 † Back pain 14 0 Respiratory, thoracic and mediastinal disorders Dyspnea # 22 3 Cough Þ 14 0 Nervous system disorders Peripheral sensory neuropathy 17 2 † Psychiatric disorders Insomnia 17 5 † Metabolism and nutrition disorders Hyperglycemia 12 9 † Hypocalcemia 11 0 Clinically relevant adverse reactions in <10% of patients who received DARZALEX FASPRO with lenalidomide and dexamethasone included:
- Musculoskeletal and connective tissue disorders: arthralgia, musculoskeletal chest pain
- Nervous system disorders: dizziness, headache, paresthesia
- Skin and subcutaneous tissue disorders: rash, pruritus
- Gastrointestinal disorders: abdominal pain
- Infections: influenza, sepsis, herpes zoster
- Metabolism and nutrition disorders: decreased appetite
- Cardiac disorders: atrial fibrillation
- General disorders and administration site conditions: chills, infusion reaction, injection site reaction
- Vascular disorders: hypotension, hypertension
Table 9 summarizes the laboratory abnormalities in patients who received DARZALEX FASPRO in PLEIADES.
Table 9: Select Hematology Laboratory Abnormalities Worsening from Baseline in Patients Who Received DARZALEX FASPRO with Lenalidomide and Dexamethasone (DARZALEX FASPRO-Rd) in PLEIADES Laboratory Abnormality DARZALEX FASPRO with Lenalidomide and Dexamethasone * All Grades
(%)Grades 3–4
(%)- *
- Denominator is based on the safety population treated with DARZALEX FASPRO-Rd (N=65).
Decreased leukocytes 94 34 Decreased lymphocytes 82 58 Decreased platelets 86 9 Decreased neutrophils 89 52 Decreased hemoglobin 45 8 In Combination with Pomalidomide and Dexamethasone
The safety of DARZALEX FASPRO with pomalidomide and dexamethasone compared to pomalidomide and dexamethasone (Pd) in patients who had received at least one prior line of therapy with lenalidomide and a proteasome inhibitor (PI) was evaluated in APOLLO [see Clinical Studies (14.2)] . Patients received DARZALEX FASPRO 1,800 mg/30,000 units administered subcutaneously once weekly from weeks 1 to 8, once every 2 weeks from weeks 9 to 24 and once every 4 weeks starting with week 25 until disease progression or unacceptable toxicity in combination with pomalidomide and dexamethasone (n=149) or pomalidomide and dexamethasone (n=150). Among patients receiving DARZALEX FASPRO-Pd, 71% were exposed for 6 months or longer and 50% were exposed for greater than one year.
Serious adverse reactions occurred in 50% of patients who received DARZALEX FASPRO-Pd. The most frequent serious adverse reactions in >5% of patients who received DARZALEX FASPRO-Pd were pneumonia (15%) and lower respiratory tract infection (12%). Fatal adverse reactions occurred in 7% of patients who received DARZALEX FASPRO-Pd.
Permanent treatment discontinuation due to an adverse reaction occurred in 2% of patients who received DARZALEX FASPRO-Pd.
The most common adverse reactions (≥20%) were fatigue, pneumonia, upper respiratory tract infection, and diarrhea.
Table 10 summarizes the adverse reactions in patients who received DARZALEX FASPRO in APOLLO.
Table 10: Adverse Reactions Reported in ≥10% of Patients and With at Least a 5% Greater Frequency in the DARZALEX FASPRO-Pd Arm in APOLLO Adverse Reaction DARZALEX FASPRO-Pd (N=149) Pd (N=150) All Grades
(%)Grades ≥3
(%)All Grades
(%)Grades ≥3
(%)Key: Pd=pomalidomide-dexamethasone - *
- Fatigue includes asthenia, and fatigue.
- †
- Only Grade 3 adverse reactions occurred.
- ‡
- Edema peripheral includes edema, edema peripheral and peripheral swelling.
- §
- Pneumonia includes atypical pneumonia, lower respiratory tract infection, pneumonia, pneumonia aspiration, pneumonia bacterial, and pneumonia respiratory syncytial viral.
- ¶
- Grade 5 adverse reactions occurred, n=3 (2.0%) in the DARZALEX FASPRO-Pd arm and n=2 (1.3%) in the Pd arm.
- #
- Upper respiratory tract infection includes nasopharyngitis, pharyngitis, respiratory syncytial virus infection, respiratory tract infection, respiratory tract infection viral, rhinitis, sinusitis, tonsillitis, upper respiratory tract infection, and viral upper respiratory tract infection.
- Þ
- Cough includes cough, and productive cough.
General disorders and administration site conditions Fatigue * 46 13 39 5 † Pyrexia 19 0 14 0 Edema peripheral ‡ 15 0 9 0 Infections Pneumonia § 38 23 ¶ 27 17 ¶ Upper respiratory infection # 36 1 † 22 2 † Gastrointestinal disorders Diarrhea 22 5 † 14 1 † Respiratory, thoracic and mediastinal disorders Cough Þ 13 0 8 0 Clinically relevant adverse reactions in <10% of patients who received DARZALEX FASPRO with pomalidomide and dexamethasone include:
- Metabolism and nutrition disorders: hypocalcemia, hypokalemia, decreased appetite, dehydration
- Nervous system disorders: peripheral sensory neuropathy, syncope, headache, paresthesia, dizziness
- Musculoskeletal and connective tissue disorders: muscle spasms, musculoskeletal chest pain, arthralgia
- Psychiatric disorders: insomnia
- Gastrointestinal disorders: nausea, abdominal pain, vomiting
- Skin and subcutaneous tissue disorders: rash, pruritus
- Cardiac disorders: atrial fibrillation
- General disorders and administration site conditions: infusion reactions, chills, injection site reaction
- Infections: urinary tract infection, influenza, hepatitis B reactivation, herpes zoster, sepsis
- Vascular disorders: hypertension, hypotension
Table 11 summarizes the laboratory abnormalities in patients who received DARZALEX FASPRO in APOLLO.
Table 11: Select Hematology Laboratory Abnormalities Worsening from Baseline in Patients Who Received DARZALEX FASPRO-Pd or Pd in APOLLO Laboratory Abnormality DARZALEX FASPRO-Pd * Pd * All Grades
(%)Grades 3–4
(%)All Grades
(%)Grades 3–4
(%)Key: Pd=pomalidomide-dexamethasone - *
- Denominator is based on number of subjects with a baseline and post-baseline laboratory value for each laboratory test: N=148 for DARZALEX FASPRO-Pd and N=149 for Pd.
Decreased neutrophils 97 84 84 63 Decreased leukocytes 95 64 82 40 Decreased lymphocytes 93 59 79 33 Decreased platelets 75 19 60 19 Decreased hemoglobin 51 16 57 15 In Combination with Carfilzomib and Dexamethasone
The safety of DARZALEX FASPRO with carfilzomib and dexamethasone was evaluated in a single-arm cohort of PLEIADES [see Clinical Studies (14.2)]. Patients received DARZALEX FASPRO 1,800 mg/30,000 units administered subcutaneously once weekly from Weeks 1 to 8, once every 2 weeks from Weeks 9 to 24 and once every 4 weeks starting with Week 25 until disease progression or unacceptable toxicity (N=66) in combination with carfilzomib and dexamethasone. Among these patients, 77% were exposed for 6 months or longer and 27% were exposed for greater than one year.
Serious adverse reactions occurred in 27% of patients who received DARZALEX FASPRO in combination with carfilzomib and dexamethasone. Fatal adverse reactions occurred in 3% of patients who received DARZALEX FASPRO in combination with carfilzomib and dexamethasone.
Permanent discontinuation of DARZALEX FASPRO due to an adverse reaction occurred in 6% of patients who received DARZALEX FASPRO.
Dosage interruptions due to an adverse reaction occurred in 46% of patients who received DARZALEX FASPRO.
The most common adverse reactions (≥20%) were upper respiratory tract infection, fatigue, insomnia, hypertension, diarrhea, cough, dyspnea, headache, pyrexia, nausea, and edema peripheral.
Table 12 summarizes the adverse reactions in patients who received DARZALEX FASPRO with carfilzomib and dexamethasone (DARZALEX FASPRO-Kd) in PLEIADES.
Table 12: Adverse Reactions (≥10%) in Patients Who Received DARZALEX FASPRO with Carfilzomib and Dexamethasone (DARZALEX FASPRO-Kd) in PLEIADES Adverse Reaction DARZALEX FASPRO-Kd
(N=66)All Grades
(%)Grade ≥3
(%)- *
- Upper respiratory tract infection includes nasopharyngitis, pharyngitis, respiratory tract infection, respiratory tract infection viral, rhinitis, sinusitis, tonsillitis, upper respiratory tract infection, viral pharyngitis, and viral upper respiratory tract infection.
- †
- Bronchitis includes bronchitis, and bronchitis viral.
- ‡
- Only Grade 3 adverse reactions occurred.
- §
- Fatigue includes asthenia, and fatigue.
- ¶
- Edema peripheral includes generalized edema, edema peripheral, and peripheral swelling.
- #
- Hypertension includes blood pressure increased, and hypertension.
- Þ
- Cough includes cough, and productive cough.
- ß
- Dyspnea includes dyspnea, and dyspnea exertional.
Infections and infestations Upper respiratory tract infection * 52 0 Bronchitis † 12 2 ‡ General disorders and administration site conditions Fatigue § 39 2 ‡ Pyrexia 21 2 ‡ Edema peripheral ¶ 20 0 Psychiatric disorders Insomnia 33 6 ‡ Vascular disorders Hypertension # 32 21 ‡ Gastrointestinal disorders Diarrhea 29 0 Nausea 21 0 Vomiting 15 0 Respiratory, thoracic and mediastinal disorders Cough Þ 24 0 Dyspnea ß 23 2 ‡ Nervous system disorders Headache 23 0 Peripheral sensory neuropathy 11 0 Musculoskeletal and connective tissue disorders Back pain 17 2 ‡ Musculoskeletal chest pain 11 0 Clinically relevant adverse reactions in <10% of patients who received DARZALEX FASPRO with carfilzomib and dexamethasone include:
- Gastrointestinal disorders: abdominal pain, constipation, pancreatitis
- Infection and infestations: pneumonia, influenza, urinary tract infection, herpes zoster, sepsis
- Metabolism and nutrition disorders: hyperglycemia, decreased appetite, hypocalcemia
- Musculoskeletal and connective tissue disorders: muscle spasms, arthralgia
- Nervous system disorders: paresthesia, dizziness, syncope
- General disorders and administration site conditions: injection site reaction, infusion reactions, chills
- Skin and subcutaneous tissue disorders: rash, pruritus
- Cardiac disorders: cardiac failure
- Vascular disorders: hypotension
Table 13 summarizes the laboratory abnormalities in patients who received DARZALEX FASPRO with carfilzomib and dexamethasone in PLEIADES.
Table 13: Select Laboratory Abnormalities (≥30%) Worsening from Baseline in Patients Who Received DARZALEX FASPRO-Kd in PLEIADES Laboratory Abnormality DARZALEX FASPRO-Kd * All Grades (%) Grades 3–4 (%) - *
- Denominator is based on the safety population treated with DARZALEX FASPRO-Kd (N=66).
Decreased platelets 88 18 Decreased lymphocytes 83 50 Decreased leukocytes 68 18 Decreased neutrophils 55 15 Decreased hemoglobin 47 6 Decreased corrected calcium 45 2 Increased alanine aminotransferase (ALT) 35 5 Monotherapy
The safety of DARZALEX FASPRO as monotherapy was evaluated in COLUMBA [see Clinical Trials (14.2)]. Patients received DARZALEX FASPRO 1,800 mg/30,000 units administered subcutaneously or daratumumab 16 mg/kg administered intravenously; each administered once weekly from weeks 1 to 8, once every 2 weeks from weeks 9 to 24 and once every 4 weeks starting with week 25 until disease progression or unacceptable toxicity. Among patients receiving DARZALEX FASPRO, 37% were exposed for 6 months or longer and 1% were exposed for greater than one year.
Serious adverse reactions occurred in 26% of patients who received DARZALEX FASPRO. Fatal adverse reactions occurred in 5% of patients. Fatal adverse reactions occurring in more than 1 patient were general physical health deterioration, septic shock, and respiratory failure.
Permanent discontinuation due to an adverse reaction occurred in 10% of patients who received DARZALEX FASPRO. Adverse reactions resulting in permanent discontinuation of DARZALEX FASPRO in more than 2 patients were thrombocytopenia and hypercalcemia.
Dosage interruptions due to an adverse reaction occurred in 26% of patients who received DARZALEX FASPRO. Adverse reactions requiring dosage interruption in >5% of patients included thrombocytopenia.
The most common adverse reaction (≥20%) was upper respiratory tract infection.
Table 14 summarizes the adverse reactions in COLUMBA.
Table 14: Adverse Reactions (≥10%) in Patients Who Received DARZALEX FASPRO or Intravenous Daratumumab in COLUMBA Adverse Reaction DARZALEX FASPRO
(N=260)Intravenous Daratumumab
(N=258)All Grades
(%)Grade ≥3
(%)All Grades
(%)Grade ≥3
(%)- *
- Upper respiratory tract infection includes acute sinusitis, nasopharyngitis, pharyngitis, respiratory syncytial virus infection, respiratory tract infection, rhinitis, rhinovirus infection, sinusitis, and upper respiratory tract infection.
- †
- Only Grade 3 adverse reactions occurred.
- ‡
- Pneumonia includes lower respiratory tract infection, lung infection, pneumocystis jirovecii pneumonia, and pneumonia.
- §
- Grade 5 adverse reactions occurred.
- ¶
- Fatigue includes asthenia, and fatigue.
- #
- Infusion reactions includes terms determined by investigators to be related to infusion.
- Þ
- Cough includes cough, and productive cough.
- ß
- Dyspnea includes dyspnea, and dyspnea exertional.
Infections Upper respiratory tract infection * 24 1 † 22 1 † Pneumonia ‡ 8 5 10 6 § Gastrointestinal disorders Diarrhea 15 1 † 11 0.4 † Nausea 8 0.4 † 11 0.4 † General disorders and administration site conditions Fatigue ¶ 15 1 † 16 2 † Infusion reactions # 13 2 † 34 5 † Pyrexia 13 0 13 1 † Chills 6 0.4 † 12 1 † Musculoskeletal and connective tissue disorders Back pain 10 2 † 12 3 † Respiratory, thoracic and mediastinal disorders Cough Þ 9 1 † 14 0 Dyspnea ß 6 1 † 11 1 † Clinically relevant adverse reactions in <10% of patients who received DARZALEX FASPRO included:
- General disorders and administration site conditions: injection site reaction, peripheral edema
- Musculoskeletal and connective tissue disorders: arthralgia, musculoskeletal chest pain, muscle spasms
- Gastrointestinal disorders: constipation, vomiting, abdominal pain
- Metabolism and nutrition disorders: decreased appetite, hyperglycemia, hypocalcemia, dehydration
- Psychiatric disorders: insomnia
- Vascular disorders: hypertension, hypotension
- Nervous system disorders: dizziness, peripheral sensory neuropathy, paresthesia
- Infections: bronchitis, influenza, urinary tract infection, herpes zoster, sepsis, hepatitis B virus reactivation
- Skin and subcutaneous tissue disorders: pruritus, rash
- Cardiac disorders: atrial fibrillation
- Respiratory, thoracic and mediastinal disorders: pulmonary edema
Table 15 summarizes the laboratory abnormalities in COLUMBA.
Table 15: Select Hematology Laboratory Abnormalities Worsening from Baseline in Patients Receiving DARZALEX FASPRO or Intravenous Daratumumab in COLUMBA Laboratory Abnormality DARZALEX FASPRO * Intravenous Daratumumab * All Grades
(%)Grades 3–4
(%)All Grades
(%)Grades 3–4
(%)- *
- Denominator is based on the safety population treated with DARZALEX FASPRO (N=260) and Intravenous Daratumumab (N=258).
Decreased leukocytes 65 19 57 14 Decreased lymphocytes 59 36 56 36 Decreased neutrophils 55 19 43 11 Decreased platelets 43 16 45 14 Decreased hemoglobin 42 14 39 16 Light Chain Amyloidosis
In Combination with Bortezomib, Cyclophosphamide and Dexamethasone
The safety of DARZALEX FASPRO with bortezomib, cyclophosphamide and dexamethasone (DARZALEX FASPRO-VCd) was evaluated in ANDROMEDA [see Clinical Studies (14.3)] . Patients received DARZALEX FASPRO 1,800 mg/30,000 units administered subcutaneously once weekly from weeks 1 to 8, once every 2 weeks from weeks 9 to 24 and once every 4 weeks starting with week 25 until disease progression or unacceptable toxicity or a maximum of 2 years. Among patients who received DARZALEX FASPRO-VCd, 74% were exposed for 6 months or longer and 32% were exposed for greater than one year.
Serious adverse reactions occurred in 43% of patients who received DARZALEX FASPRO in combination with VCd. Serious adverse reactions that occurred in at least 5% of patients in the DARZALEX FASPRO-VCd arm were pneumonia (9%), cardiac failure (8%), and sepsis (5%). Fatal adverse reactions occurred in 11% of patients. Fatal adverse reactions that occurred in more than one patient included cardiac arrest (4%), sudden death (3%), cardiac failure (3%), and sepsis (1%).
Permanent discontinuation of DARZALEX FASPRO due to an adverse reaction occurred in 5% of patients. Adverse reactions resulting in permanent discontinuation of DARZALEX FASPRO in more than one patient were pneumonia, sepsis, and cardiac failure.
Dosage interruptions (defined as dose delays or skipped doses) due to an adverse reaction occurred in 36% of patients who received DARZALEX FASPRO. Adverse reactions which required a dosage interruption in ≥3% of patients included upper respiratory tract infection (9%), pneumonia (6%), cardiac failure (4%), fatigue (3%), herpes zoster (3%), dyspnea (3%), and neutropenia (3%).
The most common adverse reactions (≥20%) were upper respiratory tract infection, diarrhea, peripheral edema, constipation, fatigue, peripheral sensory neuropathy, nausea, insomnia, dyspnea, and cough.
Table 16 below summarizes the adverse reactions in patients who received DARZALEX FASPRO in ANDROMEDA.
Table 16: Adverse Reactions (≥10%) in Patients with AL Amyloidosis Who Received DARZALEX FASPRO with Bortezomib, Cyclophosphamide and Dexamethasone (DARZALEX FASPRO-VCd) with a Difference Between Arms of >5% Compared to VCd in ANDROMEDA Adverse Reaction DARZALEX FASPRO-VCd
(N=193)VCd
(N=188)All Grades
(%)Grades 3–4
(%)All Grades
(%)Grades 3–4
(%)- *
- Upper respiratory tract infection includes laryngitis, nasopharyngitis, pharyngitis, respiratory syncytial virus infection, respiratory tract infection, respiratory tract infection viral, rhinitis, rhinovirus infection, sinusitis, tonsillitis, tracheitis, upper respiratory tract infection, upper respiratory tract infection bacterial, and viral upper respiratory tract infection.
- †
- Only Grade 3 adverse reactions occurred.
- ‡
- Pneumonia includes lower respiratory tract infection, pneumonia, pneumonia aspiration, and pneumonia pneumococcal.
- §
- Dyspnea includes dyspnea, and dyspnea exertional.
- ¶
- Cough includes cough, and productive cough.
- #
- Arrhythmia includes atrial flutter, atrial fibrillation, supraventricular tachycardia, bradycardia, arrhythmia, bradyarrhythmia, cardiac flutter, extrasystoles, supraventricular extrasystoles, ventricular arrhythmia, ventricular extrasystoles, atrial tachycardia, ventricular tachycardia
- Þ
- Injection site reactions includes terms determined by investigators to be related to daratumumab injection.
Infections Upper respiratory tract infection * 40 1 † 21 1 † Pneumonia ‡ 15 10 9 5 Gastrointestinal disorders Diarrhea 36 6 † 30 4 Constipation 34 2 † 29 0 Nervous system disorders Peripheral sensory neuropathy 31 3 † 20 2 † Respiratory, thoracic and mediastinal disorders Dyspnea § 26 4 20 4 † Cough ¶ 20 1 † 11 0 Musculoskeletal and connective tissue disorders Back pain 12 2 † 6 0 Arthralgia 10 0 5 0 Muscle spasms 10 1 † 5 0 Cardiac disorders Arrhythmia # 11 4 5 2 General disorders and administration site conditions Injection site reactions Þ 11 0 0 0 Clinically relevant adverse reactions not included in Table 16 and occurred in patients who received DARZALEX FASPRO with bortezomib, cyclophosphamide and dexamethasone included:
- Skin and subcutaneous tissue disorders: rash, pruritus
- Nervous system disorders: paresthesia
- General disorders and administration site conditions: infusion reaction, chills
- Cardiac disorders: cardiac failure 1, cardiac arrest
- Metabolism and nutrition disorders: hyperglycemia, hypocalcemia, dehydration
- Infections: bronchitis, herpes zoster, sepsis, urinary tract infection, influenza
- Vascular disorders: hypertension
- Musculoskeletal and connective tissue disorders: musculoskeletal chest pain
- Gastrointestinal disorders: pancreatitis
- Respiratory, thoracic and mediastinal disorders: pulmonary edema
Table 17 summarizes the laboratory abnormalities in patients who received DARZALEX FASPRO in ANDROMEDA.
Table 17: Select Hematology Laboratory Abnormalities Worsening from Baseline in Patients Who Received DARZALEX FASPRO with Bortezomib, Cyclophosphamide and Dexamethasone (DARZALEX FASPRO-VCd) in ANDROMEDA Laboratory Abnormality DARZALEX FASPRO-VCd VCd All Grades
(%)Grades 3–4
(%)All Grades
(%)Grades 3–4
(%)Denominator is based on the number of patients with a baseline and post-baseline laboratory value for each laboratory test, N=188 for DARZALEX FASPRO-VCd and N=186 for VCd. Decreased lymphocytes 81 54 71 46 Decreased hemoglobin 66 6 70 6 Decreased leukocytes 60 7 46 4 Decreased platelets 46 3 40 4 Decreased neutrophils 30 6 18 4
- 1
- Cardiac failure includes cardiac dysfunction, cardiac failure, cardiac failure congestive, cardiovascular insufficiency, diastolic dysfunction, pulmonary edema, and left ventricular dysfunction occurred in 11% of patients.
Cardiac Adverse Reactions in Light Chain (AL) Amyloidosis
Among patients who received DARZALEX FASPRO in combination with VCd, 72% of patients had baseline cardiac involvement with Mayo Cardiac Stage I (3%), Stage II (46%) and Stage III (51%). Serious cardiac disorders occurred in 16% of patients (8% of patients with Mayo Cardiac Stage I and II and 28% of patients with Stage III). Serious cardiac disorders in >2% of patients included cardiac failure (8%), cardiac arrest (4%) and arrhythmia (4%). Fatal cardiac disorders occurred in 10% of patients (5% of patients with Mayo Cardiac Stage I and II and 19% of patients with Stage III) who received DARZALEX FASPRO in combination with VCd. Fatal cardiac disorders that occurred in more than one patient in the DARZALEX FASPRO-VCd arm included cardiac arrest (4%), sudden death (3%), and cardiac failure (3%).
6.2 Immunogenicity
As with all therapeutic proteins, there is the potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies in the studies described below with the incidence of antibodies in other studies or to other daratumumab products or other hyaluronidase products may be misleading.
In patients with multiple myeloma and light chain (AL) amyloidosis who received DARZALEX FASPRO as monotherapy or as part of a combination therapy, less than 1% of 819 patients developed treatment-emergent anti-daratumumab antibodies.
In patients with multiple myeloma and light chain (AL) amyloidosis who received DARZALEX FASPRO as monotherapy or as part of a combination therapy, 7% of 812 patients developed treatment-emergent anti-rHuPH20 antibodies. The anti-rHuPH20 antibodies did not appear to affect daratumumab exposure. None of the patients who tested positive for anti-rHuPH20 antibodies tested positive for neutralizing antibodies.
6.3 Postmarketing Experience
The following adverse reactions have been identified with post-approval use of daratumumab. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Immune System: Anaphylactic reaction, Systemic administration reactions (including death)
Gastrointestinal: Pancreatitis
Infections: Cytomegalovirus, Listeriosis
-
7 DRUG INTERACTIONS
7.1 Effects of Daratumumab on Laboratory Tests
Interference with Indirect Antiglobulin Tests (Indirect Coombs Test)
Daratumumab binds to CD38 on RBCs and interferes with compatibility testing, including antibody screening and cross matching. Daratumumab interference mitigation methods include treating reagent RBCs with dithiothreitol (DTT) to disrupt daratumumab binding [see References (15)] or genotyping. Since the Kell blood group system is also sensitive to DTT treatment, supply K-negative units after ruling out or identifying alloantibodies using DTT-treated RBCs.
If an emergency transfusion is required, administer non-cross-matched ABO/RhD-compatible RBCs per local blood bank practices.
Interference with Serum Protein Electrophoresis and Immunofixation Tests
Daratumumab may be detected on serum protein electrophoresis (SPE) and immunofixation (IFE) assays used for monitoring disease monoclonal immunoglobulins (M protein). False positive SPE and IFE assay results may occur for patients with IgG kappa myeloma protein impacting initial assessment of complete responses by International Myeloma Working Group (IMWG) criteria. In DARZALEX FASPRO-treated patients with persistent very good partial response, where daratumumab interference is suspected, consider using a FDA-approved daratumumab-specific IFE assay to distinguish daratumumab from any remaining endogenous M protein in the patient's serum, to facilitate determination of a complete response.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
DARZALEX FASPRO can cause fetal harm when administered to a pregnant woman. The assessment of associated risks with daratumumab products is based on the mechanism of action and data from target antigen CD38 knockout animal models (see Data) . There are no available data on the use of DARZALEX FASPRO in pregnant women to evaluate drug-associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes. Animal reproduction studies have not been conducted.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
The combination of DARZALEX FASPRO and lenalidomide, thalidomide or pomalidomide is contraindicated in pregnant women, because lenalidomide, thalidomide and pomalidomide may cause birth defects and death of the unborn child. Lenalidomide, thalidomide and pomalidomide are only available through a REMS program. Refer to the lenalidomide, thalidomide or pomalidomide prescribing information on use during pregnancy.
Clinical Considerations
Fetal/Neonatal Adverse Reactions
Immunoglobulin G1 (IgG1) monoclonal antibodies are transferred across the placenta. Based on its mechanism of action, DARZALEX FASPRO may cause depletion of fetal CD38 positive immune cells and decreased bone density. Defer administering live vaccines to neonates and infants exposed to daratumumab in utero until a hematology evaluation is completed.
Data
Animal Data
DARZALEX FASPRO for subcutaneous injection contains daratumumab and hyaluronidase . Mice that were genetically modified to eliminate all CD38 expression (CD38 knockout mice) had reduced bone density at birth that recovered by 5 months of age. Data from studies using CD38 knockout animal models also suggest the involvement of CD38 in the regulation of humoral immune responses (mice), feto-maternal immune tolerance (mice), and early embryonic development (frogs).
No systemic exposure of hyaluronidase was detected in monkeys given 22,000 U/kg subcutaneously (12 times higher than the human dose) and there were no effects on embryo-fetal development in pregnant mice given 330,000 U/kg hyaluronidase subcutaneously daily during organogenesis, which is 45 times higher than the human dose.
There were no effects on pre- and post-natal development through sexual maturity in offspring of mice treated daily from implantation through lactation with 990,000 U/kg hyaluronidase subcutaneously, which is 134 times higher than the human doses.
8.2 Lactation
Risk Summary
There is no data on the presence of daratumumab and hyaluronidase in human milk, the effects on the breastfed child, or the effects on milk production. Maternal immunoglobulin G is known to be present in human milk. Published data suggest that antibodies in breast milk do not enter the neonatal and infant circulations in substantial amounts. Because of the potential for serious adverse reactions in the breastfed child when DARZALEX FASPRO is administered with lenalidomide, thalidomide or pomalidomide, advise women not to breastfeed during treatment with DARZALEX FASPRO. Refer to lenalidomide, thalidomide or pomalidomide prescribing information for additional information.
Data
Animal Data
No systemic exposure of hyaluronidase was detected in monkeys given 22,000 U/kg subcutaneously (12 times higher than the human dose) and there were no effects on post-natal development through sexual maturity in offspring of mice treated daily during lactation with 990,000 U/kg hyaluronidase subcutaneously, which is 134 times higher than the human doses.
8.3 Females and Males of Reproductive Potential
DARZALEX FASPRO can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)] .
8.4 Pediatric Use
Safety and effectiveness of DARZALEX FASPRO in pediatric patients have not been established.
8.5 Geriatric Use
Of the 291 patients who received DARZALEX FASPRO as monotherapy for relapsed and refractory multiple myeloma, 37% were 65 to <75 years of age, and 19% were 75 years of age or older. No overall differences in effectiveness of DARZALEX FASPRO have been observed between patients ≥65 years of age and younger patients . Adverse reactions that occurred at a higher frequency (≥5% difference) in patients ≥65 years of age included upper respiratory tract infection, urinary tract infection, dizziness, cough, dyspnea, diarrhea, nausea, fatigue, and peripheral edema. Serious adverse reactions that occurred at a higher frequency (≥2% difference) in patients ≥65 years of age included pneumonia.
Of the 214 patients who received DARZALEX FASPRO as combination therapy with pomalidomide and dexamethasone or DARZALEX FASPRO as combination therapy with lenalidomide and low-dose dexamethasone for relapsed and refractory multiple myeloma, 43% were 65 to <75 years of age, and 18% were 75 years of age or older. No overall differences in effectiveness were observed between patients ≥65 years (n=131) and <65 years (n=85). Adverse reactions occurring at a higher frequency (≥5% difference) in patients ≥65 years of age included fatigue, pyrexia, peripheral edema, urinary tract infection, diarrhea, constipation, vomiting, dyspnea, cough, and hyperglycemia. Serious adverse reactions occurring at a higher frequency (≥2% difference) in patients ≥65 years of age included neutropenia, thrombocytopenia, diarrhea, anemia, COVID-19, ischemic colitis, deep vein thrombosis, general physical health deterioration, pulmonary embolism, and urinary tract infection.
Of the 193 patients who received DARZALEX FASPRO as part of a combination therapy for light chain (AL) amyloidosis, 35% were 65 to <75 years of age, and 10% were 75 years of age or older. Clinical studies of DARZALEX FASPRO as part of a combination therapy for patients with light chain (AL) amyloidosis did not include sufficient numbers of patients aged 65 and older to determine whether effectiveness differs from that of younger patients. Adverse reactions that occurred at a higher frequency in patients ≥65 years of age were peripheral edema, asthenia, pneumonia and hypotension.
No clinically meaningful differences in the pharmacokinetics of daratumumab were observed in geriatric patients compared to younger adult patients [see Clinical Pharmacology (12.3)] .
-
11 DESCRIPTION
Daratumumab is an immunoglobulin G1 kappa (IgG1κ) human monoclonal antibody that binds to the CD38 antigen. Daratumumab is produced in Chinese Hamster Ovary (CHO) cells using recombinant DNA technology. The molecular weight of daratumumab is approximately 148 kDa.
Hyaluronidase (recombinant human) is an endoglycosidase used to increase the dispersion and absorption of co-administered drugs when administered subcutaneously. It is a glycosylated single-chain protein produced by Chinese Hamster Ovary cells containing a DNA plasmid encoding for a soluble fragment of human hyaluronidase (PH20). Hyaluronidase (recombinant human) has a molecular weight of approximately 61 kDa.
DARZALEX FASPRO ® (daratumumab and hyaluronidase-fihj) injection is a sterile, preservative-free, colorless to yellow, and clear to opalescent solution supplied in a single-dose vial for subcutaneous administration.
Each DARZALEX FASPRO 15 mL single-dose vial contains 1,800 mg of daratumumab and 30,000 units of hyaluronidase, L-histidine (4.9 mg), L-histidine hydrochloride monohydrate (18.4 mg), L-methionine (13.5 mg), polysorbate 20 (6 mg), sorbitol (735.1 mg), and Water for Injection, USP.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
CD38 is a transmembrane glycoprotein (48 kDa) expressed on the surface of hematopoietic cells, including clonal plasma cells in multiple myeloma and light chain (AL) amyloidosis, as well as other cell types. Surface CD38 has multiple functions, including receptor mediated adhesion, signaling, and modulation of cyclase and hydrolase activity. Daratumumab is an IgG1κ human monoclonal antibody (mAb) that binds to CD38 and inhibits the growth of CD38 expressing tumor cells by inducing apoptosis directly through Fc mediated cross linking as well as by immune-mediated tumor cell lysis through complement dependent cytotoxicity (CDC), antibody dependent cell mediated cytotoxicity (ADCC) and antibody dependent cellular phagocytosis (ADCP). A subset of myeloid derived suppressor cells (CD38+MDSCs), regulatory T cells (CD38+T regs) and B cells (CD38+B regs) are decreased by daratumumab.
Hyaluronan is a polysaccharide found in the extracellular matrix of the subcutaneous tissue. It is depolymerized by the naturally occurring enzyme hyaluronidase. Unlike the stable structural components of the interstitial matrix, hyaluronan has a half-life of approximately 0.5 days. Hyaluronidase increases permeability of the subcutaneous tissue by depolymerizing hyaluronan. In the doses administered, hyaluronidase in DARZALEX FASPRO acts locally. The effects of hyaluronidase are reversible and permeability of the subcutaneous tissue is restored within 24 to 48 hours.
12.2 Pharmacodynamics
NK cells express CD38 and are susceptible to daratumumab mediated cell lysis. Decreases in absolute counts and percentages of total NK cells (CD16+CD56+) and activated (CD16+CD56 dim) NK cells in peripheral whole blood and bone marrow were observed with DARZALEX FASPRO treatment.
12.3 Pharmacokinetics
Following the recommended dose of DARZALEX FASPRO 1,800 mg/30,000 units subcutaneously once weekly for 8 weeks, daratumumab peak concentration (C max) increased 4.8-fold and area under the curve (AUC 0–7 days) increased 5.4-fold from the 1 st dose to the 8 th dose as monotherapy. Maximum trough concentrations for DARZALEX FASPRO are typically observed at the end of the weekly dosing regimens for both monotherapy and combination therapies. The mean ± standard deviation (SD) maximum trough serum concentration (C trough) after the 8 th dose was 593 ± 306 µg/mL when DARZALEX FASPRO was administered as monotherapy and 537 ± 277 µg/mL, 526 ± 226 µg/mL, and 756 ± 276 µg/mL when DARZALEX FASPRO was administered as combination with Pd, Rd, and Kd, respectively.
Table 18 lists the observed mean (±SD) maximum trough concentrations (C trough) after the 8 th dose, simulated median (5 th–95 th percentiles) maximum C trough after the 8 th dose, simulated median (5 th–95 th percentiles) C max after the 8 th dose, and simulated median (5 th–95 th percentiles) area under the curve (AUC 0–7day) after the 8 th dose following DARZALEX FASPRO 1,800 mg/30,000 units administered subcutaneously or daratumumab 16 mg/kg administered intravenously in patients with multiple myeloma or light chain (AL) amyloidosis.
Table 18: Daratumumab Exposure for Patients with Multiple Myeloma or Light Chain (AL) Amyloidosis Parameter Intravenous Daratumumab 16 mg/kg in Patients with Multiple Myeloma * DARZALEX FASPRO 1,800 mg/30,000 units in Patients with Multiple Myeloma * DARZALEX FASPRO 1,800 mg/30,000 units in Patients with Light Chain (AL) Amyloidosis † - *
- Patients with multiple myeloma who received daratumumab monotherapy
- †
- Patients with AL amyloidosis who received daratumumab in combination with VCd
- ‡
- Geometric mean ratio between 1,800 mg SC and 16 mg/kg was 108% (90% CI: 96, 122) in patients with multiple myeloma
- §
- Source: MMY3012 Primary Analysis Clinical Study Report
- ¶
- Source: AMY3001 Primary Analysis Clinical Study Report
- #
- Source: Population Pharmacokinetics and Exposure-response Analysis Report for Subcutaneously Administered Daratumumab in Multiple Myeloma Subjects
- Þ
- Source: Population Pharmacokinetics and Exposure-response Analysis Report for Daratumumab Subcutaneous Administration for the Treatment of Subjects with AL Amyloidosis
Observed mean±SD max C trough after 8 th dose (µg/mL) 522±226 ‡,§ 593±306 ‡,§ 597±232 ¶ Simulated median (5 th–95 th percentiles) max C trough after 8 th dose (µg/mL) 472 (144–809) # 563 (177–1063) # 662 (315–1037) Þ Simulated median (5 th–95 th percentiles) C max after 8 th dose (µg/mL) 688 (369–1061) # 592 (234–1114) # 729 (390–1105) Þ Simulated median (5 th–95 th percentiles) AUC 0–7days after 8 th dose (µg/mL∙day) 4019 (1740–6370) # 4017 (1515–7564) # 4855 (2562–7522) Þ Absorption
At the recommended dose of DARZALEX FASPRO 1,800 mg/30,000 units, the absolute bioavailability is 69%, with peak concentrations occurring around 3 days (T max) in patients with multiple myeloma. Peak concentrations occurred around 4 days in patients with light chain (AL) amyloidosis.
Distribution
The estimated mean (coefficient of variation, CV) volume of distribution for the central compartment is 5.2 L (37%) and peripheral compartment was 3.8 L in patients with multiple myeloma. The estimated mean volume of distribution was 10.8 L (28%) in patients with light chain (AL) amyloidosis.
Elimination
Daratumumab is cleared by parallel linear and nonlinear saturable target mediated clearances. The estimated mean (CV%) linear clearance of daratumumab is 119 mL/day (59%) in patients with multiple myeloma and is 210 mL/day (42%) in patients with light chain (AL) amyloidosis. The estimated mean (CV%) elimination half-life associated with linear clearance is 20 days (22%) in patients with multiple myeloma and 28 days (74%) in patients with light chain (AL) amyloidosis.
Specific Populations
The following population characteristics have no clinically meaningful effect on the pharmacokinetics of daratumumab in patients administered DARZALEX FASPRO as monotherapy or as combination therapy: sex, age (33 to 92 years), renal impairment [Creatinine clearance (CLcr) 15 to 89 mL/min as determined by the Cockcroft-Gault formula], and mild hepatic impairment (total bilirubin 1 to 1.5 times ULN and AST>ULN). The effect of moderate and severe hepatic impairment on daratumumab pharmacokinetics is unknown.
Racial or Ethnic Groups
Of 190 patients with light chain (AL) amyloidosis who received DARZALEX FASPRO and had a maximum C trough after the 8 th dose, African-Americans (4%) had 24% higher daratumumab mean maximum C trough after the 8 th dose compared to that of Whites (83%) and Asians (10%) had 16% higher mean maximum C trough after the 8 th dose compared to that of Whites. The difference in exposure between that of Asians and Whites could be explained in part by differences in body size. The effect of African-American race on exposure and related safety and efficacy of daratumumab is unknown.
Body Weight
In patients with multiple myeloma who received DARZALEX FASPRO 1,800 mg/30,000 units as monotherapy, the mean maximum C trough after the 8 th dose was 12% lower in the higher body weight (BW) group (>85 kg), while the mean maximum C trough after the 8 th dose was 81% higher in the lower BW group (≤50 kg) compared to the corresponding BW groups in the intravenous daratumumab arm.
In patients with light chain (AL) amyloidosis who received DARZALEX FASPRO 1,800 mg/30,000 units in combination and had a maximum C trough after the 8 th dose, the mean maximum C trough after the 8 th dose was 22% lower in the higher BW group (>85 kg), while the mean maximum C trough was 37% higher in the lower BW group (≤50 kg) compared to the patients with body weight of 51–85 kg.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No carcinogenicity or genotoxicity studies have been conducted with daratumumab. No animal studies have been performed to evaluate the potential effects of daratumumab on reproduction or development, or to determine potential effects on fertility in males or females.
No carcinogenicity, genotoxicity, or fertility studies were conducted for recombinant human hyaluronidase. There were no effects on reproductive tissues and function and no systemic exposure of hyaluronidase in monkeys given 22,000 U/kg/week subcutaneously (12 times higher than the human dose) for 39 weeks. As hyaluronidase is a recombinant form of the endogenous human hyaluronidase, no carcinogenicity, mutagenesis, or effects on fertility are expected.
-
14 CLINICAL STUDIES
14.1 Newly Diagnosed Multiple Myeloma
In Combination with Bortezomib, Melphalan and Prednisone
The efficacy of DARZALEX FASPRO with bortezomib, melphalan and prednisone was evaluated in a single-arm cohort of PLEIADES (NCT03412565), a multi-cohort, open-label trial. Eligible patients were required to have newly diagnosed multiple myeloma who are ineligible for transplant. Patients received DARZALEX FASPRO 1,800 mg/30,000 units administered subcutaneously once weekly from weeks 1 to 6, once every 3 weeks from weeks 7 to 54 and once every 4 weeks starting with week 55 until disease progression or unacceptable toxicity; bortezomib 1.3 mg/m 2 subcutaneously twice weekly on Weeks 1, 2, 4 and 5 for the first 6-week cycle (Cycle 1; 8 doses), followed by once weekly on Weeks 1, 2, 4 and 5 for eight more 6-week cycles (Cycles 2–9; 4 doses per cycle); and melphalan 9 mg/m 2 and prednisone 60 mg/m 2 orally on Days 1 to 4 of the nine 6-week cycles (Cycles 1–9). The major efficacy outcome measure was overall response rate (ORR).
A total of 67 patients received DARZALEX FASPRO with VMP. The median age was 75 years (range: 66 to 86 years); 46% were male; 69% were White, 8% Asian, and 2% Black or African American; and 33% had ISS Stage I, 45% had ISS Stage II, and 22% had ISS Stage III disease.
Efficacy results are summarized in Table 19. The median duration of follow-up for patients was 6.9 months.
Table 19: Efficacy Results from PLEIADES in Patients Who Received DARZALEX FASPRO-VMP DARZALEX FASPRO-VMP
(N=67)CI=confidence interval - *
- Based on treated patients
Overall response rate (sCR+CR+VGPR+PR), n (%) * 59 (88%) 95% CI (%) (78%, 95%) Stringent complete response (sCR) 5 (8%) Complete response (CR) 7 (10%) Very good partial response (VGPR) 31 (46%) Partial response (PR) 16 (24%) 14.2 Relapsed/Refractory Multiple Myeloma
In Combination with Lenalidomide and Dexamethasone
The efficacy of DARZALEX FASPRO with lenalidomide and dexamethasone (DARZALEX FASPRO-Rd) was evaluated in a single-arm cohort of PLEIADES (NCT03412565), a multi-cohort, open-label trial. Patients received DARZALEX FASPRO 1,800 mg/30,000 units administered subcutaneously once weekly from weeks 1 to 8, once every 2 weeks from weeks 9 to 24 and once every 4 weeks starting with week 25 until disease progression or unacceptable toxicity with lenalidomide 25 mg once daily orally on Days 1–21 of each 28-day cycle; and dexamethasone 40 mg per week (or a reduced dose of 20 mg per week for patients >75 years or BMI <18.5). The major efficacy outcome measure was ORR.
A total of 65 patients received DARZALEX FASPRO with Rd. The median age was 69 years (range: 33 to 82 years); 69% were male; 69% were White, and 3% Black or African American; and 42% had ISS Stage I, 30% had ISS Stage II, and 28% had ISS Stage III disease. Patients had received a median of 1 prior line of therapy. A total of 52% of patients had a prior ASCT; 95% of patients received a prior PI; 59% received a prior immunomodulatory agent, including 22% who received prior lenalidomide; and 54% of patients received both a prior PI and immunomodulatory agent.
Efficacy results are summarized in Table 20. The median duration of follow-up for patients was 7.1 months.
Table 20: Efficacy Results from PLEIADES in Patients Who Received DARZALEX FASPRO-Rd DARZALEX FASPRO-Rd
(N=65)CI=confidence interval - *
- Based on treated patients
Overall response rate (sCR+CR+VGPR+PR), n (%) * 59 (91%) 95% CI (%) (81%, 97%) Stringent complete response (sCR) 4 (6%) Complete response (CR) 8 (12%) Very good partial response (VGPR) 30 (46%) Partial response (PR) 17 (26%) In Combination with Pomalidomide and Dexamethasone
The efficacy of DARZALEX FASPRO with pomalidomide and dexamethasone (DARZALEX FASPRO-Pd) versus pomalidomide and dexamethasone (Pd) alone was evaluated in APOLLO (NCT03180736), an open-label, randomized, active-controlled trial. Patients received DARZALEX FASPRO 1,800 mg/30,000 units administered subcutaneously once weekly from weeks 1 to 8, once every 2 weeks from weeks 9 to 24 and once every 4 weeks starting with week 25 until disease progression or unacceptable toxicity with pomalidomide 4 mg once daily orally on Days 1–21 of each 28-day cycle; and dexamethasone 40 mg per week (or a reduced dose of 20 mg per week for patients >75 years). The major efficacy outcome measure was progression-free survival (PFS).
A total of 304 patients were randomized: 151 to the DARZALEX FASPRO-Pd arm and 153 to the Pd arm. The median age was 67 years (range: 35 to 90); 53% were male and 89% were White, <1% were Black or African American, and <1% were Asian, and 45% had ISS Stage I, 33% had ISS Stage II, and 22% had ISS Stage III disease. Patients had received a median of 2 prior lines of therapy (range 1–5), with 11% of patients having received 1 prior line of therapy and 75% of patients having received 2–3 prior lines of therapy. All patients received a prior treatment with a PI and lenalidomide, and 56% of patients received prior ASCT. The majority of patients were refractory to lenalidomide (80%), a PI (48%), or both an immunomodulatory agent and a PI (42%).
APOLLO demonstrated an improvement in PFS in the DARZALEX FASPRO-Pd treatment group as compared to the Pd treatment group; the median PFS was 12.4 months in the DARZALEX FASPRO-Pd treatment group and 6.9 months in the Pd treatment group (HR [95% CI]: 0.63 [0.47, 0.85]; p-value = 0.0018), representing a 37% reduction in the risk of disease progression or death for patients treated with DARZALEX FASPRO-Pd versus Pd.
Figure 1: Kaplan-Meier Curve of PFS in APOLLO
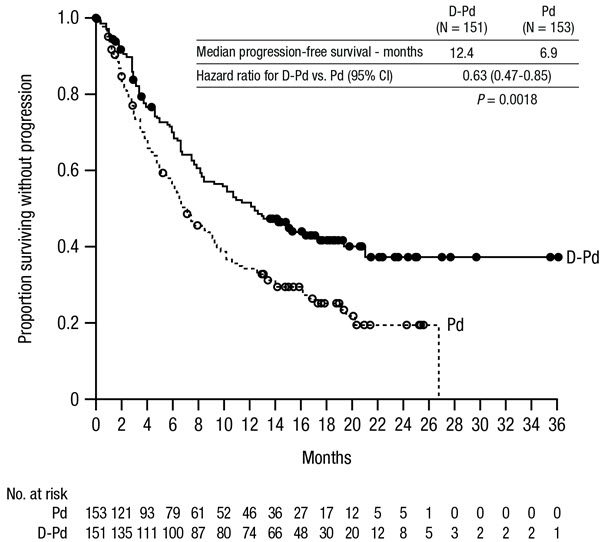
Additional efficacy results from APOLLO are presented in Table 21.
Table 21: Efficacy Results from APOLLO * DARZALEX FASPRO-Pd (n=151) Pd (n=153) Pd=pomalidomide-dexamethasone; MRD=minimal residual disease; CI=confidence interval Overall response (sCR+CR+VGPR+PR) n (%) * 104 (68.9%) 71 (46.4%) P-value † <0.0001 Stringent complete response (sCR) 14 (9.3%) 2 (1.3%) Complete response (CR) 23 (15.2%) 4 (2.6%) Very good partial response (VGPR) 40 (26.5%) 24 (15.7%) Partial response (PR) 27 (17.9%) 41 (26.8%) MRD negativity rate ‡, § n (%) 13 (8.6%) 3 (2.0%) 95% CI (%) (4.7%, 14.3%) (0.4%, 5.6%) P-value ¶ 0.0102 MRD negativity rate in patients with CR or better § Number of patients with CR or better N=37 N=6 MRD negativity rate n (%) 13 (35.1%) 3 (50.0%) 95% CI (%) (20.2%, 52.5%) (11.8%, 88.2%) In responders, the median time to response was 1 month (range: 0.9 to 9.1 months) in the DARZALEX FASPRO-Pd group and 1.9 months (range: 0.9 to 17.3 months) in the Pd group. The median duration of response had not been reached in the DARZALEX FASPRO-Pd group (range: 1 to 34.9+ months) and was 15.9 months (range: 1+ to 24.8 months) in the Pd group.
With a median follow-up of 16.9 months, 99 deaths were observed; 48 in the DARZALEX FASPRO-Pd group and 51 in the Pd group. Median OS was not reached for either treatment group.
In Combination with Carfilzomib and Dexamethasone
The efficacy of DARZALEX FASPRO with carfilzomib and dexamethasone (DARZALEX FASPRO-Kd) was evaluated in a single-arm cohort of PLEIADES (NCT03412565), a multi-cohort, open-label trial. This cohort enrolled patients with relapsed or refractory multiple myeloma excluding patients with left ventricular ejection fraction (LVEF) less than 40%, myocardial infarction within 6 months, uncontrolled cardiac arrhythmia, or uncontrolled hypertension (systolic blood pressure >159 mmHg or diastolic >99 mmHg despite optimal treatment). Patients received DARZALEX FASPRO 1,800 mg/30,000 units administered subcutaneously once weekly from Weeks 1 to 8, once every 2 weeks from Weeks 9 to 24 and once every 4 weeks starting with Week 25 until disease progression or unacceptable toxicity with carfilzomib administered by IV infusion at a dose of 20 mg/m 2 on Cycle 1 Day 1 and if a dose of 20 mg/m 2 was tolerated, carfilzomib was administered at a dose of 70 mg/m 2 as a 30-minute IV infusion, on Cycle 1 Day 8 and Day 15, and then Day 1, 8 and 15 of each cycle and dexamethasone 40 mg per week (or a reduced dose of 20 mg per week for patients ≥75 years or BMI <18.5). The major efficacy outcome measure was ORR.
A total of 66 patients received DARZALEX FASPRO with Kd. The median age was 61 years (range: 42 to 84); 52% were male; 73% were White and 3% Black or African American; and 68% had ISS Stage I, 18% had ISS Stage II, and 14% had ISS Stage III disease. A total of 79% of patients had a prior ASCT; 91% of patients received a prior PI. All patients received 1 prior line of therapy with exposure to lenalidomide and 62% of patients were refractory to lenalidomide.
Efficacy results are summarized in Table 22. At a median follow-up of 9.2 months, the median duration of response had not been reached and an estimated 85.2% (95% CI: 72.5, 92.3) maintained response for at least 6 months and 82.5% (95% CI: 68.9, 90.6) maintained response for at least 9 months.
Table 22: Efficacy Results from PLEIADES in Patients Who Received DARZALEX FASPRO-Kd DARZALEX FASPRO-Kd
(N=66)CI=confidence interval - *
- Based on treated patients
Overall response rate (sCR+CR+VGPR+PR), n (%) * 56 (84.8%) 95% CI (%) (73.9%, 92.5%) Stringent complete response (sCR) 11 (16.7%) Complete response (CR) 14 (21.2%) Very good partial response (VGPR) 26 (39.4%) Partial response (PR) 5 (7.6%) Monotherapy
The efficacy of DARZALEX FASPRO as monotherapy was evaluated in COLUMBA (NCT03277105), an open-label, randomized, non-inferiority study. Eligible patients were required to have relapsed or refractory multiple myeloma who had received at least 3 prior lines of therapy including a proteasome inhibitor and an immunomodulatory agent or who were double-refractory to a proteasome inhibitor and an immunomodulatory agent. Patients were randomized to receive DARZALEX FASPRO 1,800 mg/30,000 units administered subcutaneously or daratumumab 16 mg/kg administered intravenously; each administered once weekly from weeks 1 to 8, once every 2 weeks from weeks 9 to 24 and once every 4 weeks starting with week 25 until unacceptable toxicity or disease progression. The major efficacy outcome measures were ORR by the IMWG response criteria and maximum C trough at pre-dose Cycle 3 Day 1 [see Clinical Pharmacology (12.3)] . Randomization was stratified by body weight, myeloma type, and number of prior lines of therapy.
A total of 522 patients were randomized: 263 to the DARZALEX FASPRO arm and 259 to the intravenous daratumumab arm. The median age was 67 years (range: 33 to 92 years); 55% were male; and 78% were White, 14% Asian, and 3% Black or African American. The median weight was 73 kg (range: 29 to 138). Patients had received a median of 4 prior lines of therapy. A total of 51% of patients had a prior ASCT; 100% of patients received both a PI and an immunomodulatory agent. Forty-nine percent of patients were refractory both a PI and an immunomodulatory agent. Eighty-two percent of patients were refractory to their last line of prior systemic therapy.
The results show that DARZALEX FASPRO 1,800 mg/30,000 units administered subcutaneously is non-inferior to daratumumab 16 mg/kg administered intravenously in terms of ORR and maximum trough concentration [see Clinical Pharmacology (12.3)] . Median progression-free survival was 5.6 months in the DARZALEX FASPRO arm and 6.1 months in the intravenous daratumumab arm. ORR results are provided in Table 23.
Table 23: Efficacy Results from COLUMBA DARZALEX FASPRO
(N=263)Intravenous Daratumumab
(N=259)- *
- Based on intent-to-treat population.
Overall response (sCR+CR+VGPR+PR), n (%) * 108 (41%) 96 (37%) 95% CI (%) (35%, 47%) (31%, 43%) Ratio of response rates (95% CI) 1.11 (0.89, 1.37) CR or better, n (%) 5 (1.9%) 7 (2.7%) Very good partial response (VGPR) 45 (17%) 37 (14%) Partial response (PR) 58 (22%) 52 (20%) 14.3 Light Chain Amyloidosis
In Combination with Bortezomib, Cyclophosphamide and Dexamethasone
The efficacy of DARZALEX FASPRO with VCd was evaluated in ANDROMEDA (NCT03201965), an open-label, randomized, active-controlled trial. Eligible patients were required to have newly diagnosed light chain (AL) amyloidosis with at least one affected organ, measurable hematologic disease, Cardiac Stage I-IIIA (based on European Modification of Mayo 2004 Cardiac Stage), and NYHA Class I-IIIA. Patients with NYHA Class IIIB and IV were excluded. Patients were randomized to receive bortezomib 1.3 mg/m 2 administered subcutaneously, cyclophosphamide 300 mg/m 2 (max dose 500 mg) administered orally or intravenously, and dexamethasone 40 mg (or a reduced dose of 20 mg for patients >70 years or body mass index <18.5 or who have hypervolemia, poorly controlled diabetes mellitus or prior intolerance to steroid therapy) administered orally or intravenously on Days 1, 8, 15, and 22 of each 28-day cycle with or without DARZALEX FASPRO 1,800 mg/30,000 units subcutaneously once weekly from weeks 1 to 8, once every 2 weeks from weeks 9 to 24 and once every 4 weeks starting with week 25 until disease progression or a maximum of two years. When DARZALEX FASPRO and dexamethasone were administered on the same day, dexamethasone 20 mg was administered before DARZALEX FASPRO with the remaining dose of dexamethasone administered after DARZALEX FASPRO if applicable. The major efficacy outcome measure was confirmed hematologic complete response (HemCR) rate based on Consensus Criteria as determined by the Independent Review Committee (negative serum and urine immunofixation, involved free light chain level decrease to less than the upper limit of normal, and normal free light chain ratio). Randomization was stratified by Cardiac Stage (European Modification of Mayo 2004 Cardiac Stage) countries that typically offer autologous stem cell transplant (ASCT) for patients with light chain (AL) amyloidosis, and renal function.
A total of 388 patients were randomized: 195 to DARZALEX FASPRO-VCd and 193 to VCd. The median patient age was 64 years (range: 34 to 87 years); 58% were male; 76% White, 17% Asian, and 3% Black or African American; 23% had light chain (AL) amyloidosis Cardiac Stage I, 40% had Stage II, and 37% had Stage IIIA. The median number of organs involved was 2 (range: 1–6) and 66% of patients had 2 or more organs involved. Vital organ involvement was: cardiac 71%, renal 59% and hepatic 8%. The majority (79%) of patients had lambda free light chain disease.
Efficacy results are summarized in Table 24.
Table 24: Efficacy Results from ANDROMEDA * DARZALEX FASPRO-VCd
(n=195)VCd
(n=193)VCd=bortezomib-cyclophosphamide-dexamethasone Hematologic complete response (HemCR), n (%) 82 (42%) 26 (13%) p-value † <0.0001 Very good partial response (VGPR), n (%) 71 (36%) 69 (36%) Partial response (PR), n (%) 26 (13%) 53 (27%) Hematologic VGPR or better (HemCR + VGPR), n (%) 153 (78%) 95 (49%) Major organ deterioration progression-free survival ‡, Hazard ratio with 95% CI 0.58 (0.37, 0.92) The median time to HemCR was 59 days (range: 8 to 299 days) in the DARZALEX FASPRO-VCd arm and 59 days (range: 16 to 340 days) in the VCd arm. The median time to VGPR or better was 17 days (range: 5 to 336 days) in the DARZALEX FASPRO-VCd arm and 25 days (range: 8 to 171 days) in the VCd arm. The median duration of HemCR had not been reached in either arm.
The median follow-up for the study is 11.4 months. Overall survival (OS) data were not mature. A total of 56 deaths were observed [N=27 (13.8%) DARZALEX FASPRO-VCd vs. N=29 (15%) VCd group].
- 15 REFERENCES
-
16 HOW SUPPLIED/STORAGE AND HANDLING
DARZALEX FASPRO ® (daratumumab and hyaluronidase-fihj) injection is a sterile, preservative-free, colorless to yellow, and clear to opalescent solution for subcutaneous use supplied as individually packaged single-dose vials providing 1,800 mg of daratumumab and 30,000 units of hyaluronidase per 15 mL (NDC 57894-503-01).
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Hypersensitivity and Other Administration Reactions
Advise patients to seek immediate medical attention for any of the following signs and symptoms of systemic administration-related reactions: itchy, runny or blocked nose; chills, nausea, throat irritation, cough, headache, shortness of breath or difficulty breathing, and blurred vision [see Warnings and Precautions (5.1)] .
Cardiac Toxicity in Patients with Light Chain (AL) Amyloidosis
Advise patients to immediately contact their healthcare provider if they have signs or symptoms of cardiac adverse reactions [see Warnings and Precautions (5.2)] .
Neutropenia
Advise patients to contact their healthcare provider if they have a fever [see Warnings and Precautions (5.3)] .
Thrombocytopenia
Advise patients to contact their healthcare provider if they have bruising or bleeding [see Warnings and Precautions (5.4)] .
Embryo-Fetal Toxicity
Advise pregnant women of the potential hazard to a fetus. Advise females of reproductive potential to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions (5.5), Use in Specific Populations (8.1, 8.3)] .
Advise females of reproductive potential to avoid becoming pregnant during treatment with DARZALEX FASPRO and for 3 months after the last dose [see Use in Specific Populations (8.1, 8.3)] .
Advise patients that lenalidomide, thalidomide and pomalidomide have the potential to cause fetal harm and have specific requirements regarding contraception, pregnancy testing, blood and sperm donation, and transmission in sperm. Lenalidomide, thalidomide and pomalidomide are only available through a REMS program [see Use in Specific Populations (8.1, 8.3)] .
Interference with Laboratory Tests
Advise patients to inform their healthcare provider, including personnel at blood transfusion centers, that they are taking DARZALEX FASPRO, in the event of a planned transfusion [see Warnings and Precautions (5.6)] .
Advise patients that DARZALEX FASPRO can affect the results of some tests used to determine complete response in some patients and additional tests may be needed to evaluate response [see Warnings and Precautions (5.7)] .
Hepatitis B Virus (HBV) Reactivation
Advise patients to inform healthcare providers if they have ever had or might have a hepatitis B infection and that DARZALEX FASPRO could cause hepatitis B virus to become active again [see Adverse Reactions (6.1)] .
- SPL UNCLASSIFIED SECTION
-
PATIENT PACKAGE INSERT
PATIENT INFORMATION
DARZALEX (Dar'-zah-lex) FASPRO ® (Fas-pro)
(daratumumab and hyaluronidase-fihj)
injection, for subcutaneous useThis Patient Information has been approved by the U.S. Food and Drug Administration. Revised: 12/2022 DARZALEX FASPRO may be used with other medicines called lenalidomide, thalidomide or pomalidomide. You should also read the Medication Guide that comes with lenalidomide, thalidomide or pomalidomide if you use DARZALEX FASPRO with these medicines. What is DARZALEX FASPRO?
DARZALEX FASPRO is a prescription medicine used to treat adult patients with multiple myeloma:- in combination with the medicines bortezomib, melphalan and prednisone, in people with newly diagnosed multiple myeloma who cannot receive a type of stem cell transplant that uses their own stem cells (autologous stem cell transplant).
- in combination with the medicines lenalidomide and dexamethasone in people with newly diagnosed multiple myeloma who cannot receive a type of stem cell transplant that uses their own stem cells (autologous stem cell transplant) and in people whose multiple myeloma has come back or did not respond to treatment, who have received at least one prior medicine to treat multiple myeloma.
- in combination with the medicines bortezomib, thalidomide, and dexamethasone in newly diagnosed people who are eligible to receive a type of stem cell transplant that uses their own stem cells (autologous stem cell transplant).
- in combination with the medicines bortezomib and dexamethasone in people who have received at least one prior medicine to treat multiple myeloma.
- in combination with the medicines pomalidomide and dexamethasone in people who have received at least one prior medicine including lenalidomide and a proteasome inhibitor to treat multiple myeloma.
- in combination with the medicines carfilzomib and dexamethasone in people whose multiple myeloma has come back or did not respond to treatment who have received one to three prior medicines to treat multiple myeloma.
- alone in people who have received at least three prior medicines, including a proteasome inhibitor and an immunomodulatory agent, or did not respond to a proteasome inhibitor and an immunomodulatory agent.
It is not known if DARZALEX FASPRO is safe and effective in children.Do not receive DARZALEX FASPRO if you have a history of a severe allergic reaction to daratumumab, hyaluronidase or any of the ingredients in DARZALEX FASPRO. See the end of this leaflet for a complete list of ingredients in DARZALEX FASPRO. Before you receive DARZALEX FASPRO, tell your healthcare provider about all of your medical conditions, including if you: - have a history of breathing problems.
- have had shingles (herpes zoster).
- have ever had or might now have a hepatitis B infection as DARZALEX FASPRO could cause hepatitis B virus to become active again. Your healthcare provider will check you for signs of this infection before, during and for some time after treatment with DARZALEX FASPRO. Tell your healthcare provider right away if you get worsening tiredness or yellowing of your skin or white part of your eyes.
- are pregnant or plan to become pregnant. DARZALEX FASPRO may harm your unborn baby. Tell your healthcare provider right away if you become pregnant or think that you may be pregnant during treatment with DARZALEX FASPRO.
- Females who are able to become pregnant should use an effective method of birth control (contraception) during treatment and for 3 months after your last dose of DARZALEX FASPRO. Talk to your healthcare provider about birth control methods that you can use during this time.
- Before starting DARZALEX FASPRO in combination with lenalidomide, thalidomide or pomalidomide, females and males must agree to the instructions in the lenalidomide, thalidomide or pomalidomide REMS program.
- The lenalidomide, thalidomide and pomalidomide REMS have more information about effective methods of birth control, pregnancy testing, and blood donation for females who can become pregnant.
- For males who have female partners who can become pregnant, there is information in the lenalidomide, thalidomide and pomalidomide REMS about sperm donation and how lenalidomide, thalidomide and pomalidomide can pass into human semen.
- are breastfeeding or plan to breastfeed. It is not known if DARZALEX FASPRO passes into your breast milk. You should not breastfeed during treatment with DARZALEX FASPRO. Talk to your healthcare provider about the best way to feed your baby during treatment with DARZALEX FASPRO.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.How will I receive DARZALEX FASPRO? - DARZALEX FASPRO may be given alone or together with other medicines used to treat multiple myeloma.
- DARZALEX FASPRO will be given to you by your healthcare provider as an injection under the skin, in the stomach area (abdomen).
- DARZALEX FASPRO is injected over 3 to 5 minutes.
- Your healthcare provider will decide the time between doses as well as how many treatments you will receive.
- Your healthcare provider will give you medicines before each dose of DARZALEX FASPRO and after each dose of DARZALEX FASPRO to help reduce the risk of serious allergic reactions and other reactions due to release of certain substances by your body (systemic).
What are the possible side effects of DARZALEX FASPRO?
DARZALEX FASPRO may cause serious reactions, including:- Serious allergic reactions and other severe injection-related reactions. Serious allergic reactions and reactions due to release of certain substances by your body (systemic) that can lead to death, can happen with DARZALEX FASPRO. Your healthcare provider may temporarily stop or completely stop treatment with DARZALEX FASPRO if you have a serious reaction. Tell your healthcare provider or get medical help right away if you get any of these symptoms during or after an injection of DARZALEX FASPRO.
- shortness of breath or trouble breathing
- dizziness or lightheadedness (hypotension)
- cough
- wheezing
- heart beating faster than usual
- low oxygen in the blood (hypoxia)
- throat tightness or irritation
- runny or stuffy nose
- headache
- itching
- high blood pressure
- eye pain
- nausea
- vomiting
- chills
- fever
- chest pain
- blurred vision
- Injection site reactions. Skin reactions at or near the injection site (local), including injection site reactions, can happen with DARZALEX FASPRO. Symptoms at the site of injection may include itching, swelling, bruising, pain, rash, bleeding, or redness of the skin. These reactions sometimes happen more than 24 hours after an injection of DARZALEX FASPRO.
- Heart problems in people with light chain (AL) amyloidosis. Heart problems, in some cases fatal, have occurred. Your healthcare provider will monitor you closely during treatment with DARZALEX FASPRO. Call your healthcare provider right away if you get any of the following symptoms: chest pain, feeling faint, swollen legs, shortness of breath, or abnormal heart rhythm.
- Decreases in blood cell counts. DARZALEX FASPRO can decrease white blood cell counts which help fight infections and blood cells called platelets which help to clot blood. Decreases in blood cell counts are common with DARZALEX FASPRO, but can be severe. Your healthcare provider will check your blood cell counts during treatment with DARZALEX FASPRO. Tell your healthcare provider if you develop fever or have signs of bruising or bleeding.
- Changes in blood tests. DARZALEX FASPRO can affect the results of blood tests to match your blood type. These changes can last for up to 6 months after your final dose of DARZALEX FASPRO. Your healthcare provider will do blood tests to match your blood type before you start treatment with DARZALEX FASPRO. Tell all of your healthcare providers that you are being treated with DARZALEX FASPRO before receiving blood transfusions.
The most common side effects of DARZALEX FASPRO used in combination therapy include:- tiredness
- nausea
- diarrhea
- shortness of breath
- trouble sleeping
- headache
- fever
- cough
- muscle spasms
- back pain
- vomiting
- high blood pressure
- cold-like symptoms (upper respiratory infection)
- nerve damage causing tingling, numbness, or pain
- constipation
- lung infection (pneumonia)
- swollen hands, ankles, or feet
- decreased red blood cell counts
These are not all the possible side effects of DARZALEX FASPRO.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.General information about the safe and effective use of DARZALEX FASPRO.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. You can ask your pharmacist or healthcare provider for information about DARZALEX FASPRO that is written for health professionals.What are the ingredients in DARZALEX FASPRO?
Active ingredient: daratumumab and hyaluronidase-fihj
Inactive ingredients: L-histidine, L-histidine hydrochloride monohydrate, L-methionine, polysorbate 20, sorbitol, and water for injection.
Manufactured by: Janssen Biotech, Inc., Horsham, PA 19044, USA
U.S. License Number 1864
For patent information: www.janssenpatents.com
©2021 Janssen Pharmaceutical Companies
For more information, call 1-800-526-7736 or go to www.DARZALEXFASPRO.com. - PRINCIPAL DISPLAY PANEL - 15 mL Vial Box
-
INGREDIENTS AND APPEARANCE
DARZALEX FASPRO
daratumumab and hyaluronidase-fihj (human recombinant) injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:57894-503 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DARATUMUMAB (UNII: 4Z63YK6E0E) (DARATUMUMAB - UNII:4Z63YK6E0E) DARATUMUMAB 1800 mg in 15 mL HYALURONIDASE (HUMAN RECOMBINANT) (UNII: 743QUY4VD8) (HYALURONIDASE (HUMAN RECOMBINANT) - UNII:743QUY4VD8) HYALURONIDASE (HUMAN RECOMBINANT) 30000 U in 15 mL Inactive Ingredients Ingredient Name Strength METHIONINE (UNII: AE28F7PNPL) HISTIDINE MONOHYDROCHLORIDE MONOHYDRATE (UNII: X573657P6P) POLYSORBATE 20 (UNII: 7T1F30V5YH) SORBITOL (UNII: 506T60A25R) WATER (UNII: 059QF0KO0R) HISTIDINE (UNII: 4QD397987E) Product Characteristics Color yellow (colorless to yellow) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:57894-503-01 1 in 1 BOX 05/01/2020 1 15 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761145 05/01/2020 Labeler - Janssen Biotech, Inc. (099091753) Establishment Name Address ID/FEI Business Operations Janssen Biotech, Inc. 038978363 api manufacture(57894-503) , analysis(57894-503) Establishment Name Address ID/FEI Business Operations Eurofins Lancaster Laboratories, Inc 069777290 analysis(57894-503) Establishment Name Address ID/FEI Business Operations Charles River Laboratories 078495006 analysis(57894-503) Establishment Name Address ID/FEI Business Operations Bioreliance Corporation 147227730 analysis(57894-503) Establishment Name Address ID/FEI Business Operations Catalent Indiana, LLC 172209277 api manufacture(57894-503) Establishment Name Address ID/FEI Business Operations Biogen (Denmark) Manufacturing ApS 307258082 api manufacture(57894-503) Establishment Name Address ID/FEI Business Operations Janssen Biologics B.V. 409612918 analysis(57894-503) Establishment Name Address ID/FEI Business Operations Cilag AG 483237103 manufacture(57894-503) , analysis(57894-503) , pack(57894-503) , label(57894-503) Establishment Name Address ID/FEI Business Operations Janssen Vaccines Corp. 688272061 manufacture(57894-503) , pack(57894-503) , analysis(57894-503) Establishment Name Address ID/FEI Business Operations Janssen Sciences Ireland UC 986030167 analysis(57894-503)


