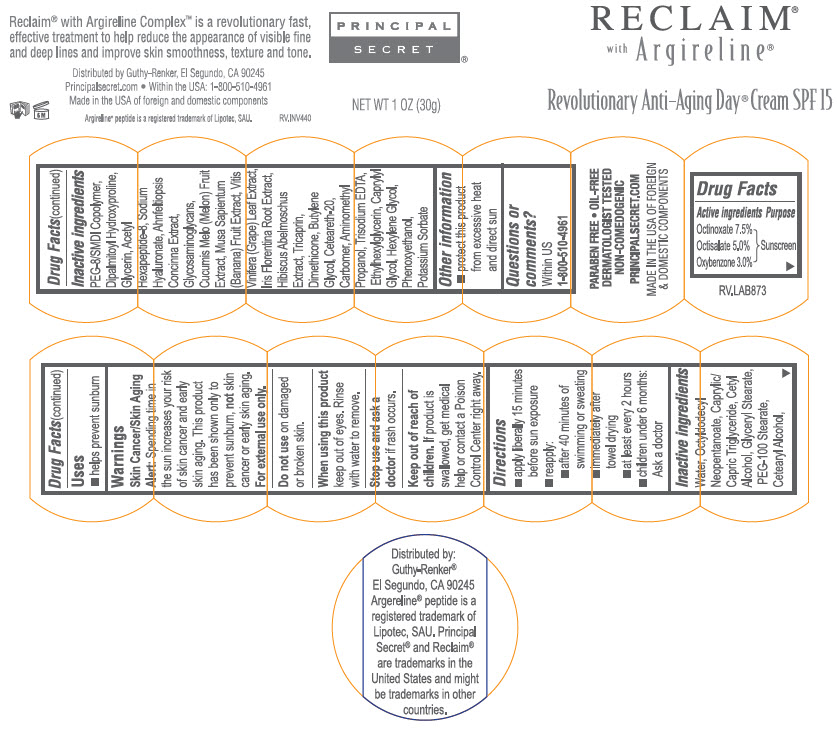
Label: RECLAIM REVOLUTIONARY ANTI-AGING DAY SPF15- octinoxate, octisalate, and oxybenzone cream
- NDC Code(s): 70605-042-07, 70605-042-15, 70605-042-43
- Packager: Guthy-Renker LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated April 8, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
-
Inactive ingredients
Water, Octyldodecyl Neopentanoate, Caprylic/Capric Triglyceride, Cetyl Alcohol, Glyceryl Stearate, PEG-100 Stearate, Cetearyl Alcohol, PEG-8/SMDI Copolymer, Dipalmitoyl Hydroxyproline, Glycerin, Acetyl Hexapeptide-8, Sodium Hyaluronate, Ahnfeltia Concinna Extract, Glycosaminoglycans, Cucumis Melo (Melon) Fruit Extract, Musa Sapientum (Banana) Fruit Extract, Vitis Vinifera (Grape) Leaf Extract, Iris Florentina Extract, Hibiscus Abelmoschuus Extract, Tricaprin, Dimethicone, Butylene Glycol, Cetereath-20, Carbomer, Aminomethyl Propanol, Trisodium EDTA, Ethylhexylglycerin, Caprylyl Glycol, Hexylene Glycol, Phenoxyethanol, Potassium Sorbate.
- Other information
- Questions or comments?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 30g Jar Label
-
INGREDIENTS AND APPEARANCE
RECLAIM REVOLUTIONARY ANTI-AGING DAY SPF15
octinoxate, octisalate, and oxybenzone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70605-042 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 7.5 g in 100 g Octisalate (UNII: 4X49Y0596W) (Octisalate - UNII:4X49Y0596W) Octisalate 5 g in 100 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 3 g in 100 g Inactive Ingredients Ingredient Name Strength OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CETYL ALCOHOL (UNII: 936JST6JCN) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PEG-100 STEARATE (UNII: YD01N1999R) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) DIPALMITOYL HYDROXYPROLINE (UNII: E6AHA53N1H) PEG-8/SMDI COPOLYMER (UNII: CCX72L6NY6) GLYCERIN (UNII: PDC6A3C0OX) ACETYL HEXAPEPTIDE-8 (UNII: L4EL31FWIL) HYALURONATE SODIUM (UNII: YSE9PPT4TH) AHNFELTIOPSIS CONCINNA (UNII: SMF2K46G8D) MUSKMELON (UNII: ZV095H5633) BANANA (UNII: 4AJZ4765R9) VITIS VINIFERA LEAF (UNII: R1H893D80E) IRIS X GERMANICA NOTHOVAR. FLORENTINA ROOT (UNII: M30XO5X4XD) TRICAPRIN (UNII: O1PB8EU98M) DIMETHICONE (UNII: 92RU3N3Y1O) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) POLYOXYL 20 CETOSTEARYL ETHER (UNII: YRC528SWUY) TROLAMINE (UNII: 9O3K93S3TK) EDETATE TRISODIUM (UNII: 420IP921MB) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) CAPRYLYL GLYCOL (UNII: 00YIU5438U) HEXYLENE GLYCOL (UNII: KEH0A3F75J) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) PHENOXYETHANOL (UNII: HIE492ZZ3T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70605-042-07 15 g in 1 JAR; Type 0: Not a Combination Product 12/30/2017 12/31/2021 2 NDC:70605-042-43 30 g in 1 JAR; Type 0: Not a Combination Product 12/30/2017 3 NDC:70605-042-15 45 g in 1 JAR; Type 0: Not a Combination Product 12/30/2017 12/31/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 12/30/2017 Labeler - Guthy-Renker LLC (948861877)