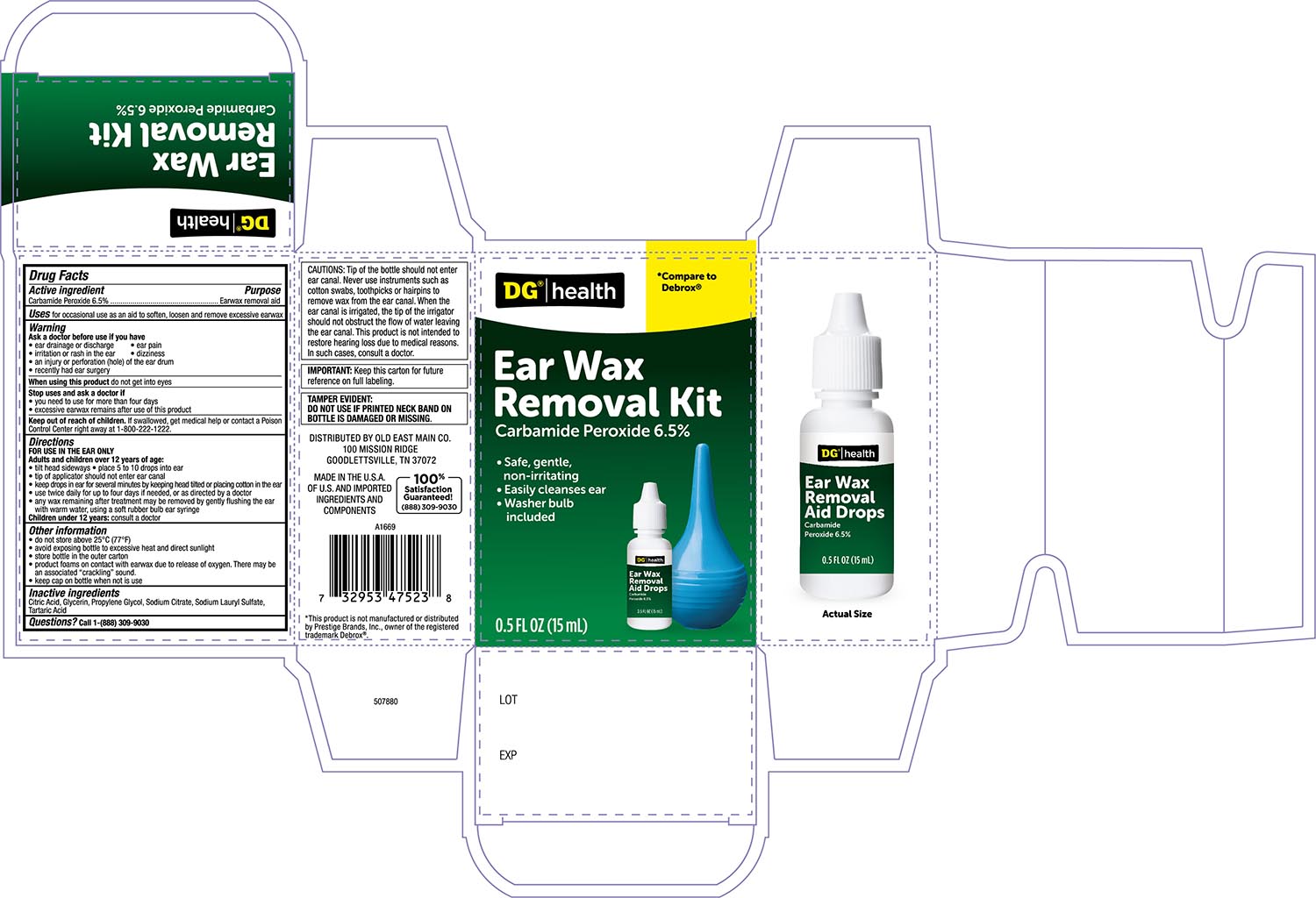
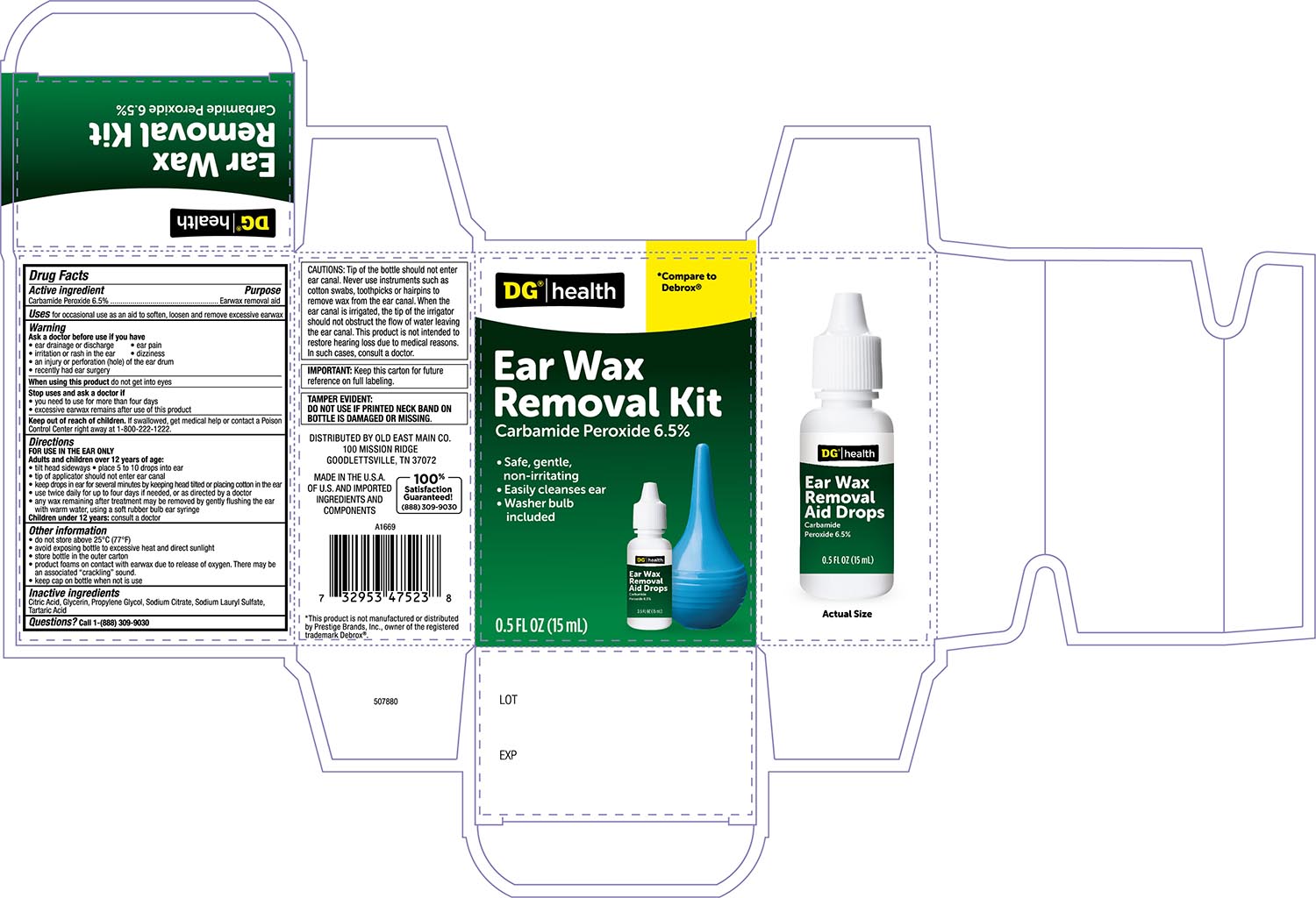
Label: DG HEALTH EARWAX REMOVAL KIT- carbamide peroxide 6.5% solution/ drops
- NDC Code(s): 75712-301-14
- Packager: DOLLAR GENERAL CORPORATION
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 7, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
- Warnings
- Stop Use and ask a Doctor if
- When using this product
- Keep out of the reach of children
-
Directions FOR USE IN THE EAR ONLY
- Adults and children over 12 years of age:
- Tilt head sideways and place 5 to 10 drops into ear.
- Tip of applicator should not enter ear canal.
- Keep drops in ear for several minutes by keeping head tilted or placing cotton in the ear.
- Use twice daily for up to 4 days if needed, or as directed by a doctor.
- Any earwax remaining after treatment may be removed by gently flushing the ear with warm water, using a soft rubber bulb ear syringe.
- When the ear canal is irrigated, the tip of the ear syringe should not obstruct the flow of water leaving the ear canal.
- Children under 12 years: consult a doctor.
- Other information
- Inactive ingredients:
- Principal Display Panel -Bottle label 0.5 FL OZ
- Principal Display Panel- Carton label 0.5 FLOZ
-
INGREDIENTS AND APPEARANCE
DG HEALTH EARWAX REMOVAL KIT
carbamide peroxide 6.5% solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:75712-301 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CARBAMIDE PEROXIDE (UNII: 31PZ2VAU81) (HYDROGEN PEROXIDE - UNII:BBX060AN9V) CARBAMIDE PEROXIDE 65 mg in 1 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SODIUM CITRATE (UNII: 1Q73Q2JULR) SODIUM LAURYL SULFATE (UNII: 368GB5141J) TARTARIC ACID (UNII: W4888I119H) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75712-301-14 1 in 1 CARTON 11/01/2023 1 15 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M014 11/01/2023 Labeler - DOLLAR GENERAL CORPORATION (006946172)