Label: LITTLE REMEDIES NEW BABY ESSENTIALS- acetaminophen, simethicone, zinc oxide kit
- NDC Code(s): 63029-615-01
- Packager: Medtech Products Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated March 11, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
-
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if your child takes ▪ more than 5 doses in 24 hours, which is the maximum daily amount ▪ with other drugs containing acetaminophen.
Sore Throat Warning: If sore throat is severe, persists for more than 2 days, or is accompanied or followed by fever, headache, rash, nausea or vomiting, consult a doctor promptly.
Allergy Alert: Acetaminophen may cause severe skin reactions. Symptoms may include: ▪ skin reddening ▪ blisters ▪ rash. If a skin reaction occurs, stop use and seek medical help right away.
Do not use
- with any other products containing acetaminophen (prescription or non-prescription).
If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if your child is allergic to acetaminophen or any of the inactive ingredients in this product.
Stop use and ask a doctor if
- new symptoms occur
- fever gets worse or lasts more than 3 days
- pain gets worse or lasts more than 5 days
- redness or swelling is present. These could be signs of a serious condition.
Keep out of reach of children.
Overdose Warning: Taking more than the recommended dose (overdose) can cause serious liver damage. In case of overdose, get medical help or contact a Poison Control Center (1-800-222-1222) right away. Prompt medical attention is critical even if you do not notice any signs or symptoms.
-
Directions
This product does not contain directions or complete warnings for adult use.
-
Shake well before using
- Find the right dose on the chart on the chart below. If possible, use weight to determine dose; otherwise, use age.
- Only use the enclosed AccuSafe® syringe. Do not use any other syringe, dropper, spoon or dosing device when giving this medicine to your child.
- Remove cap, insert syringe to flow restrictor and invert bottle
- Pull back syringe until filled to the prescribed level and slowly dispense the liquid into your child’s mouth (towards the inner cheek)
- If needed, repeat dose every 4 hours while symptoms last
- Do not give more than 5 times in any 24-hour period (see overdose warning)
- Do not give more than 5 days unless directed by a doctor
- Replace cap tightly to maintain child resistance
mL = milliliter
Weight (lbs) Age (yrs) Dose (mL) Under 24 under 2 Ask a doctor 24-35 2-3 5 mL -
Shake well before using
- Other information
-
Inactive ingredients
benzoic acid, carboxymethylcellulose, glycerin, natural grape flavor, microcrystalline cellulose, sucrose, sodium benzoate, water, xanthan gum
FOR DIAPER RASH
BOUDREAUX’S BUTT PASTE ® Diaper Rash OintmentDrug Facts Active ingredient Purpose Zinc oxide, 16%...................................................Skin protectant Uses - Helps treat and prevent diaper rash
- Protects chafed skin due to diaper rash and helps seal out wetness
Warnings For external use only When using this product avoid contact with the eyes Stop use and ask a doctor if condition worsens or does not improve after 7 days Keep out of the reach of children to prevent accidental ingestion If swallowed, seek medical help or call Poison Control Center (1-800-222-1222) immediately Directions - Change wet and soiled diaper immediately
- Cleanse the diaper area and allow to dry
- Apply ointment liberally and as often as necessary with each diaper change and especially when exposed to wet diapers for a prolonged period of time, such as bedtime
Other information
- Store at room temperature 20°- 27°C (68°- 80°F)
- Remove seal from jar before use
- Use with infants, children and adults
- Will stain clothing and fabric
Inactive ingredients castor oil, mineral oil, paraffin, peruvian balsam, white petrolatum Questions? 1-855-785-2888 www.buttpaste.com FOR TUMMYSTM
GAS RELIEF DROPS Simethicone / Antigas
Drug Facts
- Active ingredient (in each 0.3 mL)
- Purpose
- Uses
- Warnings
-
Directions
■ shake well before using
■ only use enclosed syringe; fill to prescribed level and dispense liquid slowly into child’s mouth, toward inner cheek
■ all dosages may be repeated as needed, after meals and at bedtime or as directed by a physician. Do not exceed 12 doses per day.
■ dosage can also be mixed with 1 oz. of cool water, infant formula or other suitable liquids
■ for best results, clean syringe after each use and replace original cap
If possible, use weight to determine dose; otherwise use age.
mL = milliliters
Age (yrs) Weight (lbs) Dose Newborns &
Infants under 2 yearsunder 24 0.3 mL Children 2 years and over 24 and over 0.6 mL - Other information
-
Inactive ingredients
carmellose sodium,citric acid, microcrystalline cellulose, natural strawberry flavor, propylene glycol, purified water, sodium benzoate, sucralose, xanthan gum
FOR TUMMYSTM
GRIPE WATER
Supplement Facts
Serving Size: 5 mL
Servings per Container: about 12Amount per Serving % Daily Value Zingiber officinale (Ginger) Root Extract 5 mg † Foeniculum vulgare (Fennel) Seed Extract 4mg † †Daily Value not established -
Other ingredients
purified water, agave, glycerin, natural ginger flavor, potassium sorbate, citric acid, xanthan gum
Suggested Use Age Dose 2 to 4 weeks 2.5 mL 1 to 6 months 5 mL 6 months and older 10 mL Adults 30 mL SHAKE WELL. May be taken directly using the dispenser included. May be given up to six times in a 24 hour period. If necessary to repeat dosage, wait a minimum of 30 minutes. Do not use if safety seal is broken or missing.
- Uses
- Warnings
- Directions
- Ingredients:
-
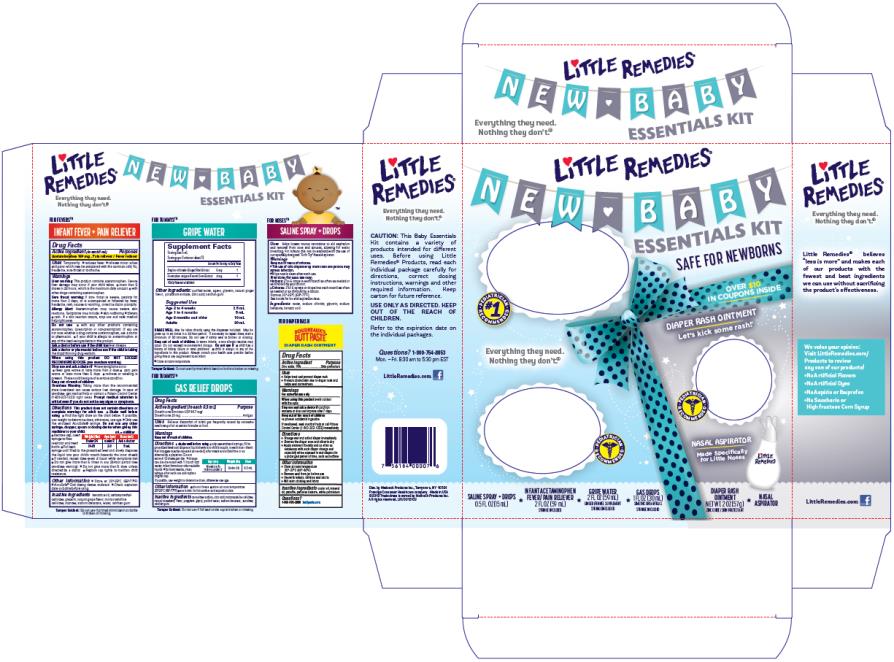
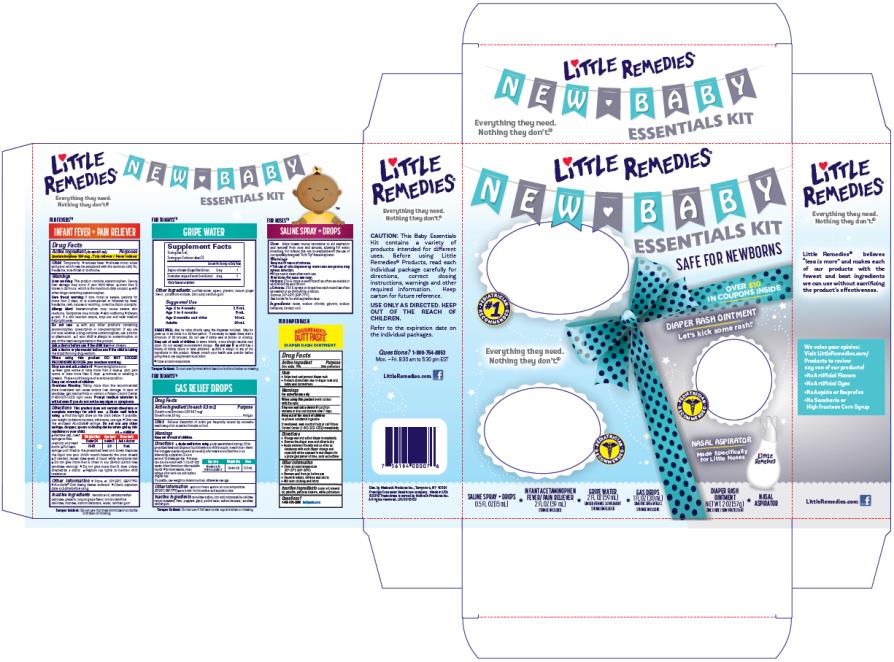
PRINCIPAL DISPLAY PANEL
Little Remedies®
New Baby Essentials Kit
INFANT FEVER/PAIN RELIEVER
ACETAMINOPHEN / FEVER/PAIN RELIEVER
2 FL OZ (59 mL)
Syringe Included
GAS DROPS
SIMETHICONE /ANTIGAS
1 FL OZ (30 mL)
Syringe Included
DIAPER RASH OINTMENT
ZINC OXIDE / SKIN PROTECTANT
2 OZ (57 g)
SALINE SPRAY + DROPS
0.5 FL OZ (15 mL)
GRIPE WATER
GINGER / FENNEL SUPPLEMENT
2 FL OZ (59 mL)
Syringe Included
Nasal Aspirator
-
INGREDIENTS AND APPEARANCE
LITTLE REMEDIES NEW BABY ESSENTIALS
acetaminophen, simethicone, zinc oxide kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63029-615 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63029-615-01 1 in 1 CARTON; Type 0: Not a Combination Product 02/15/2017 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE 59 mL Part 2 1 BOTTLE 30 mL Part 3 1 TUBE 57 g Part 4 1 BOTTLE 59 mL Part 5 1 BOTTLE 15 mL Part 1 of 5 LITTLE REMEDIES INFANT FEVER AND PAIN RELIEVER
acetaminophen liquidProduct Information Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 160 mg in 5 mL Inactive Ingredients Ingredient Name Strength BENZOIC ACID (UNII: 8SKN0B0MIM) CARBOXYMETHYLCELLULOSE (UNII: 05JZI7B19X) GLYCERIN (UNII: PDC6A3C0OX) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SUCROSE (UNII: C151H8M554) SODIUM BENZOATE (UNII: OJ245FE5EU) WATER (UNII: 059QF0KO0R) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color WHITE Score Shape Size Flavor BERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 59 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part343 02/15/2017 Part 2 of 5 LITTLE REMEDIES RELIEF
simethicone liquidProduct Information Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 20 mg in 0.3 mL Inactive Ingredients Ingredient Name Strength CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SUCRALOSE (UNII: 96K6UQ3ZD4) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color WHITE Score Shape Size Flavor BERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part332 02/15/2017 Part 3 of 5 BOUDREAUXS
zinc oxide creamProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 16 g in 113 g Inactive Ingredients Ingredient Name Strength CASTOR OIL (UNII: D5340Y2I9G) MINERAL OIL (UNII: T5L8T28FGP) PARAFFIN (UNII: I9O0E3H2ZE) BALSAM PERU (UNII: 8P5F881OCY) PETROLATUM (UNII: 4T6H12BN9U) Product Characteristics Color WHITE (Beige) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 57 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part347 02/15/2017 Part 4 of 5 GRIPE WATER
ginger and fennel liquidProduct Information Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GINGER (UNII: C5529G5JPQ) (GINGER - UNII:C5529G5JPQ) GINGER 5 mg in 5 mL FENNEL (UNII: 557II4LLC3) (FENNEL - UNII:557II4LLC3) FENNEL 4 mg in 5 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) AGAVE TEQUILANA JUICE (UNII: GVG8G0207O) GLYCERIN (UNII: PDC6A3C0OX) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color WHITE (clear) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 59 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date DIETARY SUPPLEMENT 02/15/2017 Part 5 of 5 SALINE
other baby products sprayProduct Information Route of Administration NASAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR WATER (UNII: 059QF0KO0R) INGR SODIUM CHLORIDE (UNII: 451W47IQ8X) INGR GLYCERIN (UNII: PDC6A3C0OX) INGR SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) INGR POTASSIUM PHOSPHATE, UNSPECIFIED FORM (UNII: B7862WZ632) INGR BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) INGR EDETATE DISODIUM (UNII: 7FLD91C86K) Product Characteristics Color WHITE (clear) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 15 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date COSMETIC 02/15/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED DRUG OTHER 02/15/2017 Labeler - Medtech Products Inc. (122715688)