Label: XYLAZINE powder
- NDC Code(s): 64189-9001-0, 64189-9001-1, 64189-9001-2, 64189-9001-3
- Packager: GRINDEKS Joint Stock Company
- Category: BULK INGREDIENT - ANIMAL DRUG
- DEA Schedule: None
- Marketing Status: bulk ingredient
Drug Label Information
Updated November 16, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- WARNINGS AND PRECAUTIONS
- STORAGE AND HANDLING
-
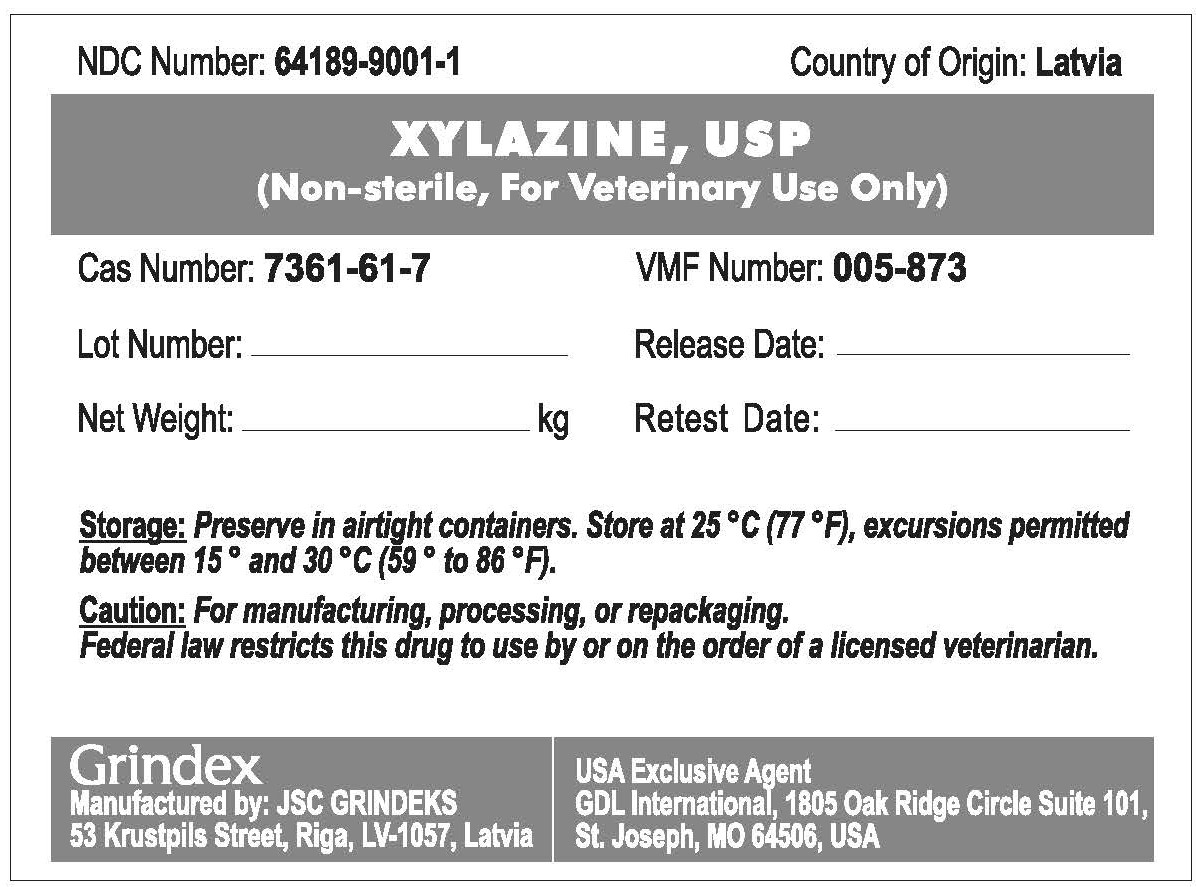
PRINCIPAL DISPLAY PANEL
NDC Number: 64189-9001-1 Country of Origin: Latvia
XYLAZINE, USP
(Non-sterile, For Veterinary Use Only)
Cas Number: 7361-61-7 VMF Number: 005-873
Lot Number:_______________ Release Date:____________
Net Weight:_______________k Retest Date:_____________
Storage: Preserve in tight containers. Store at 25°C (77°F), excursions permitted between 15° and 30°C (59° to 86°F).
Caution: For manufacturing, processing, or repackaging. Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Grindex
Manufactured by: JSC GRINDEKS
53 Krustpils Street, Riga, LV-1057, Latvia
USA Exclusive Agent
GDL International, 1805 Oak Ridge Circle Suite 101
St. Joseph, MO 64506, USA
-
INGREDIENTS AND APPEARANCE
XYLAZINE
xylazine powderProduct Information Product Type Item Code (Source) NDC:64189-9001 Route of Administration NOT APPLICABLE Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength XYLAZINE (UNII: 2KFG9TP5V8) (XYLAZINE - UNII:2KFG9TP5V8) XYLAZINE 1 kg in 1 kg Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64189-9001-0 1 in 1 DRUM 1 1 kg in 1 BAG 2 NDC:64189-9001-1 1 in 1 DRUM 2 5 kg in 1 BAG 3 NDC:64189-9001-2 1 in 1 DRUM 3 10 kg in 1 BAG 4 NDC:64189-9001-3 1 in 1 DRUM 4 30 kg in 1 BAG Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date bulk ingredient 10/04/2006 Labeler - GRINDEKS Joint Stock Company (644702888) Establishment Name Address ID/FEI Business Operations GRINDEKS Joint Stock Company 644702888 api manufacture