Label: HEALING ECZEMA BALM- oatmeal cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 72257-001-15, 72257-001-80 - Packager: PROVINCE APOTHECARY INC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 1, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
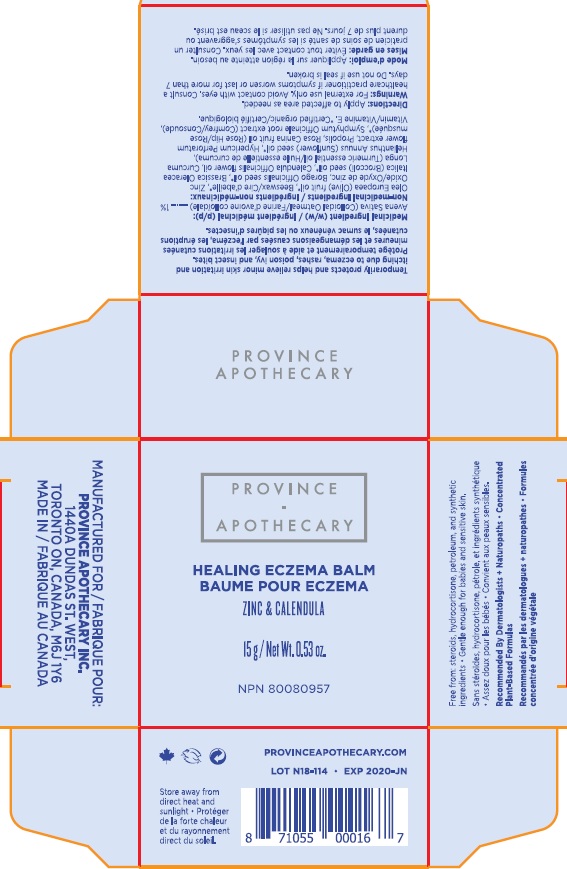
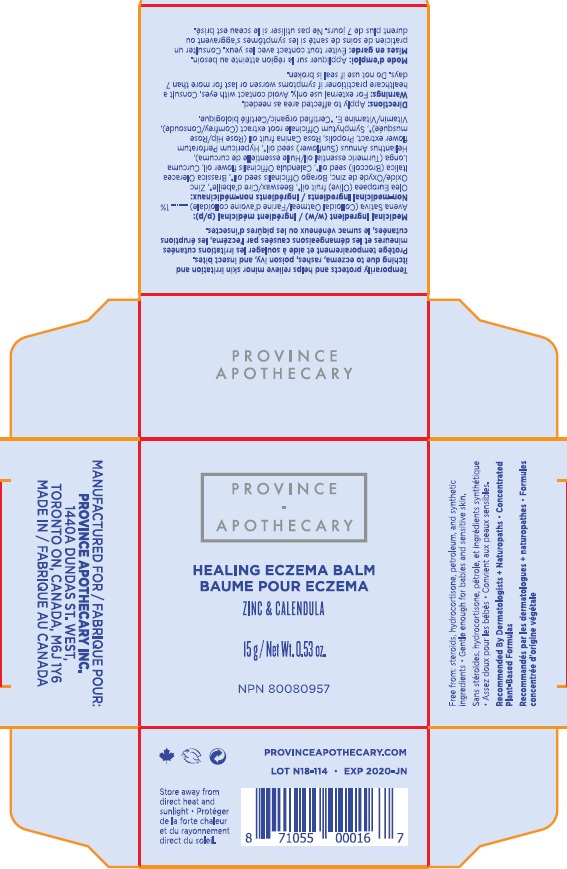
- HEALING ECZEMA BALM
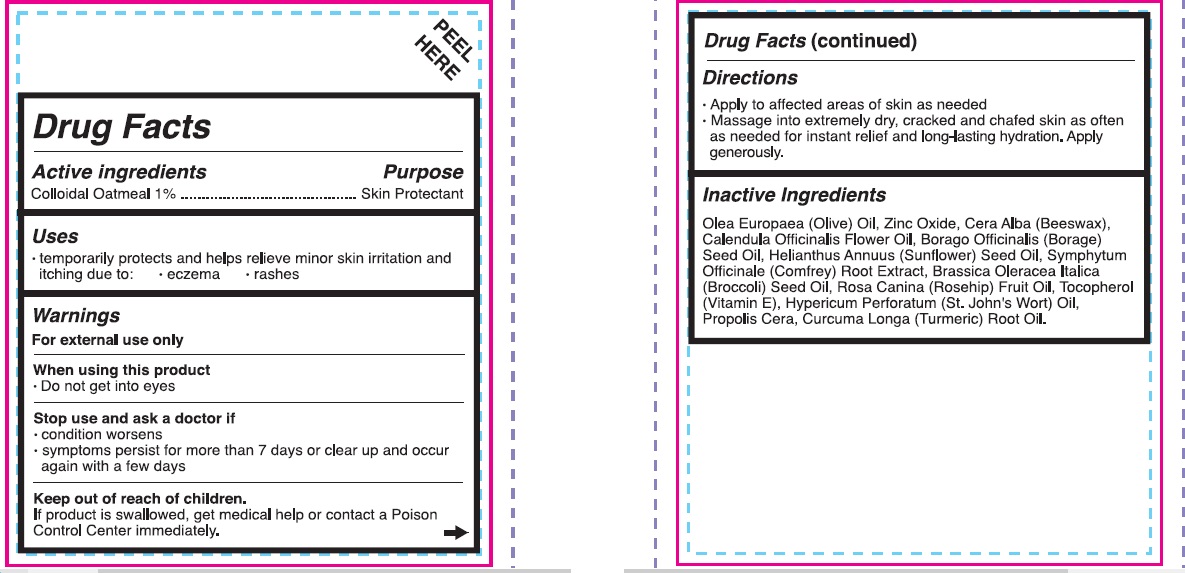
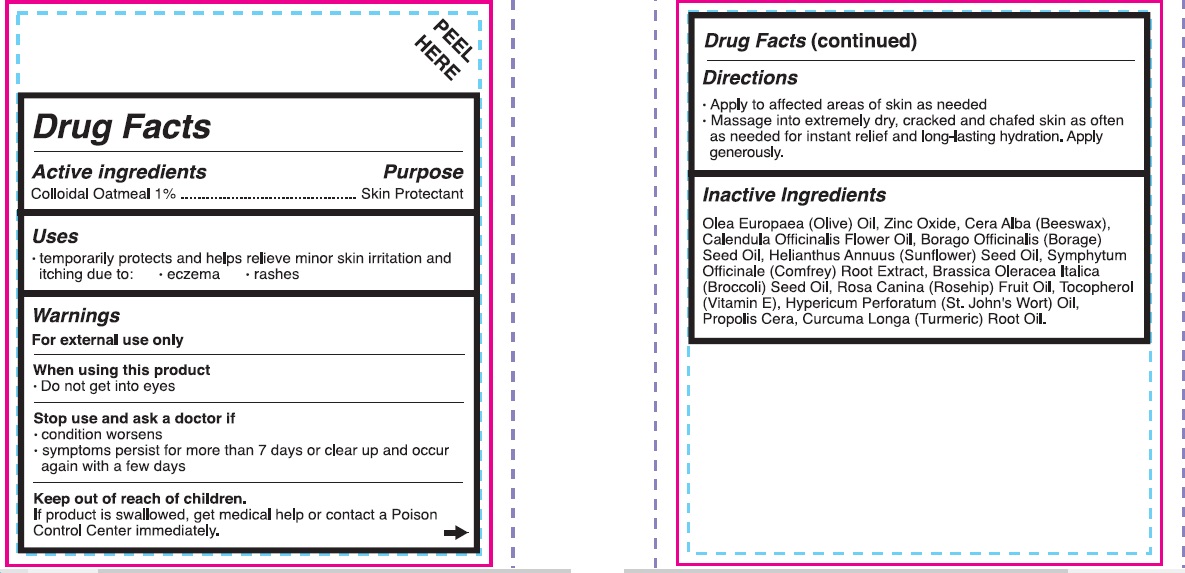
- Active Ingredients
- Purpose
- Uses
- Warnings
- Directions
-
Inactive ingredients
- Olea Europaea (Olive) Oil, Zinc Oxide, Cera Alba (Beesawax), Calendula Officinalis Flower Oil, Borago Officinalis (Borage) Seed Oil, Helianthus Annuus (Sunflower) Seed Oil, Symphytum Officinale (Comfrey) Root Extract, Brassica Oleracea Italica (Broccoli) Seed Oul, Rosa Canina (Rosehip) Fruit Oil, Tocopherol (Vitamin E), Hypericum Perforatum (St.John's Wort) Oil, Propolis Cera, Curcuma Longa (Turmeric) Root Oil.
- HEALING ECZEMA BALM 15g (72257-001-15)
- ECZEMA BALM 80g (72257-001-80)
- Drug Facts
-
INGREDIENTS AND APPEARANCE
HEALING ECZEMA BALM
oatmeal creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72257-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OATMEAL (UNII: 8PI54V663Y) (OATMEAL - UNII:8PI54V663Y) OATMEAL 1 g in 100 g Inactive Ingredients Ingredient Name Strength OLIVE OIL (UNII: 6UYK2W1W1E) ZINC OXIDE (UNII: SOI2LOH54Z) YELLOW WAX (UNII: 2ZA36H0S2V) CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) BORAGO OFFICINALIS SEED (UNII: 2GXJ790US0) SUNFLOWER OIL (UNII: 3W1JG795YI) COMFREY ROOT (UNII: M9VVZ08EKQ) BROCCOLI SEED OIL (UNII: SY01LVD4G4) ROSA CANINA FRUIT OIL (UNII: CR7307M3QZ) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) HYPERICUM PERFORATUM (UNII: XK4IUX8MNB) PROPOLIS WAX (UNII: 6Y8XYV2NOF) TURMERIC OIL (UNII: 6KGS8SP16U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72257-001-15 1 in 1 BOX 09/11/2018 1 15 g in 1 JAR; Type 0: Not a Combination Product 2 NDC:72257-001-80 1 in 1 BOX 09/11/2018 2 80 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part347 09/11/2018 Labeler - PROVINCE APOTHECARY INC (201115232) Registrant - PROVINCE APOTHECARY INC (201115232)