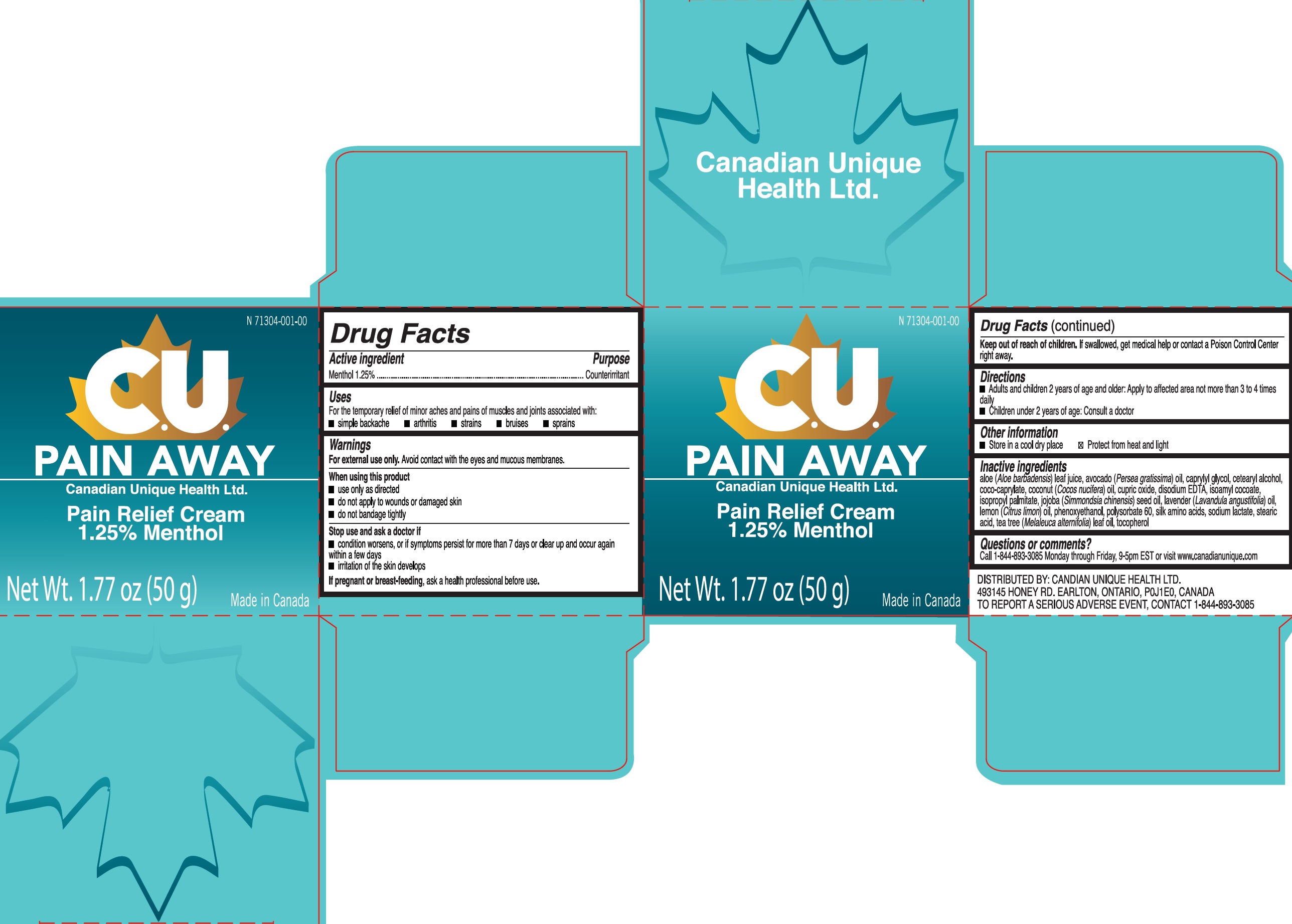
Label: CU PAIN AWAY PAIN RELIEF- menthol cream
- NDC Code(s): 71304-001-00
- Packager: Canadian Unique Health Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 28, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Uses
-
Warnings
For external use only. Avoid contact with the eyes and mucous membranes.
When using this product
- use only as directed
- do not apply to wounds or damaged skin
- do not bandage tightly
- Directions
- Other information
-
Inactive ingredients
aloe (Aloe barbadensis) leaf juice, avocado (Persea gratissima) oil, caprylyl glycol, cetearyl alcohol, coco-caprylate, coconut (Cocos nucifera) oil, cupric oxide, disodium EDTA, isoamyl cocoate, isopropyl palmitate, jojoba (Simmondsia chinensis) seed oil, lavender (Lavandula angustifolia) oil, lemon (Citrus limon) oil, phenoxyethanol, polysorbate 60, silk amino acids, sodium lactate, stearic acid, tea tree (Melaleuca alternifolia) leaf oil, tocopherol
- Questions or comments?
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
CU PAIN AWAY PAIN RELIEF
menthol creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71304-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 12.5 mg in 1 g Inactive Ingredients Ingredient Name Strength ALOE (UNII: V5VD430YW9) AVOCADO OIL (UNII: 6VNO72PFC1) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) COCO-CAPRYLATE (UNII: 4828G836N6) COCONUT OIL (UNII: Q9L0O73W7L) CUPRIC OXIDE (UNII: V1XJQ704R4) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) ISOAMYL COCOATE (UNII: 14OG46E98E) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) JOJOBA OIL (UNII: 724GKU717M) LAVENDER OIL (UNII: ZBP1YXW0H8) LEMON OIL (UNII: I9GRO824LL) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYSORBATE 60 (UNII: CAL22UVI4M) AMINO ACIDS, SILK (UNII: V0L00EX1IA) SODIUM LACTATE (UNII: TU7HW0W0QT) STEARIC ACID (UNII: 4ELV7Z65AP) TOCOPHEROL (UNII: R0ZB2556P8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71304-001-00 1 in 1 BOX 03/08/2017 1 50 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 03/08/2017 Labeler - Canadian Unique Health Ltd. (203413273)