Label: PVP SCRUB SOLUTION- povidone iodine solution
- NDC Code(s): 68599-3501-1, 68599-3501-2, 68599-3501-5, 68599-3501-6
- Packager: McKesson Medical-Surgical Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated May 21, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- PURPOSE
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- ACTIVE INGREDIENT
- INDICATIONS & USAGE
-
DOSAGE & ADMINISTRATION
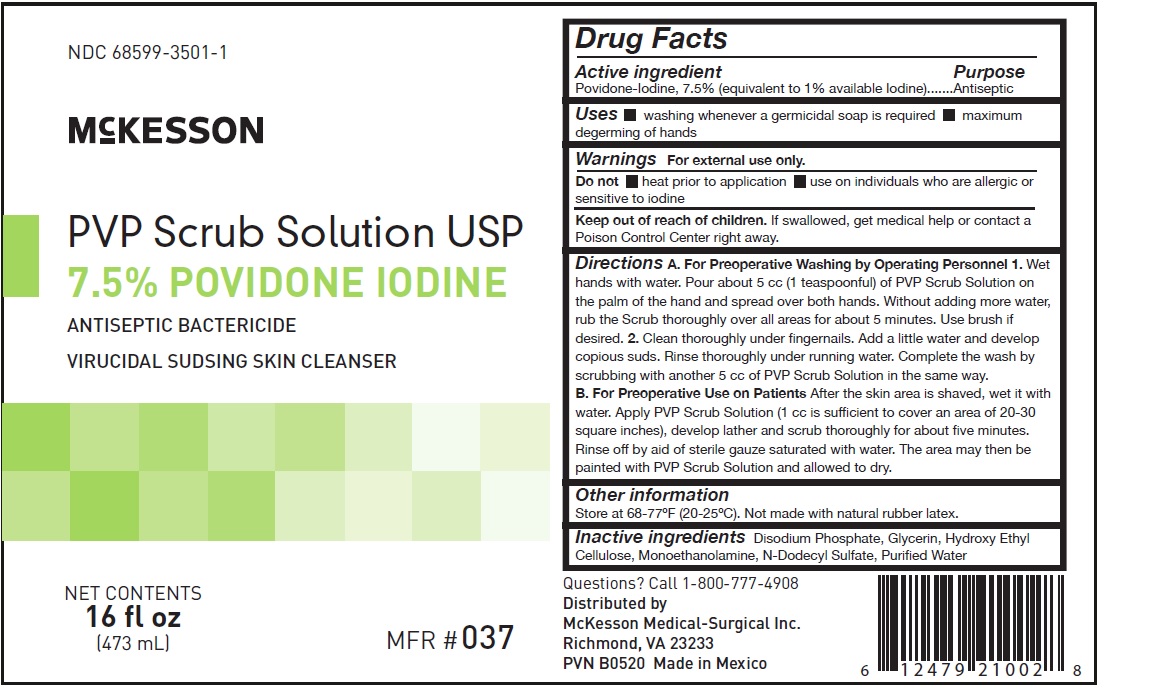
A. For Preoperative Washing by Operating Personnel
1. Wet hands with water. Pour about 5 cc (1 teaspoonful) of PVP Scrub Solution on the palm of the hand and spread over both hands. Without adding more water, rub the Scrub thoroughly over all areas for about five minutes. Use brush if desired.
2. Clean thoroughly under fingernails. Add a litle water and develop copious suds. Rinse thoroughly under running water. Complete the wash by scrubbing with another 5 cc of PVP Scrub Solution in the same way.
B. For Preoperative Use on Patients
After the skin area is shaved, wet it with water. Apply PVP Scrub Solution (1 cc is sufficient to cover an area of 20-30 square inches), develop lather and scrub thoroughly for about five minutes. Rinse off by aid of sterile gauze saturated with water. The area may then be painted with PVP Scrub Solution and allowed to dry.
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PVP SCRUB SOLUTION
povidone iodine solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68599-3501 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POVIDONE-IODINE (UNII: 85H0HZU99M) (IODINE - UNII:9679TC07X4) IODINE 7.5 mg Inactive Ingredients Ingredient Name Strength HYDROXYETHYL CELLULOSE (5000 MPA.S AT 1%) (UNII: X70SE62ZAR) GLYCERIN (UNII: PDC6A3C0OX) MONOETHANOLAMINE (UNII: 5KV86114PT) LAURYL SULFATE (UNII: DIQ16UC154) WATER (UNII: 059QF0KO0R) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68599-3501-2 12 in 1 CASE 03/03/2017 1 NDC:68599-3501-1 29.57 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:68599-3501-6 4 in 1 CASE 03/03/2017 2 NDC:68599-3501-5 15141.6 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333E 03/03/2017 Labeler - McKesson Medical-Surgical Inc. (023904428) Establishment Name Address ID/FEI Business Operations Degassa 812771980 manufacture(68599-3501)