Label: LA CREME P- octinoxate avobenzone titanium dioxide cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 42248-133-02 - Packager: Zenith Medicosm SL
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated May 23, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
-
Description
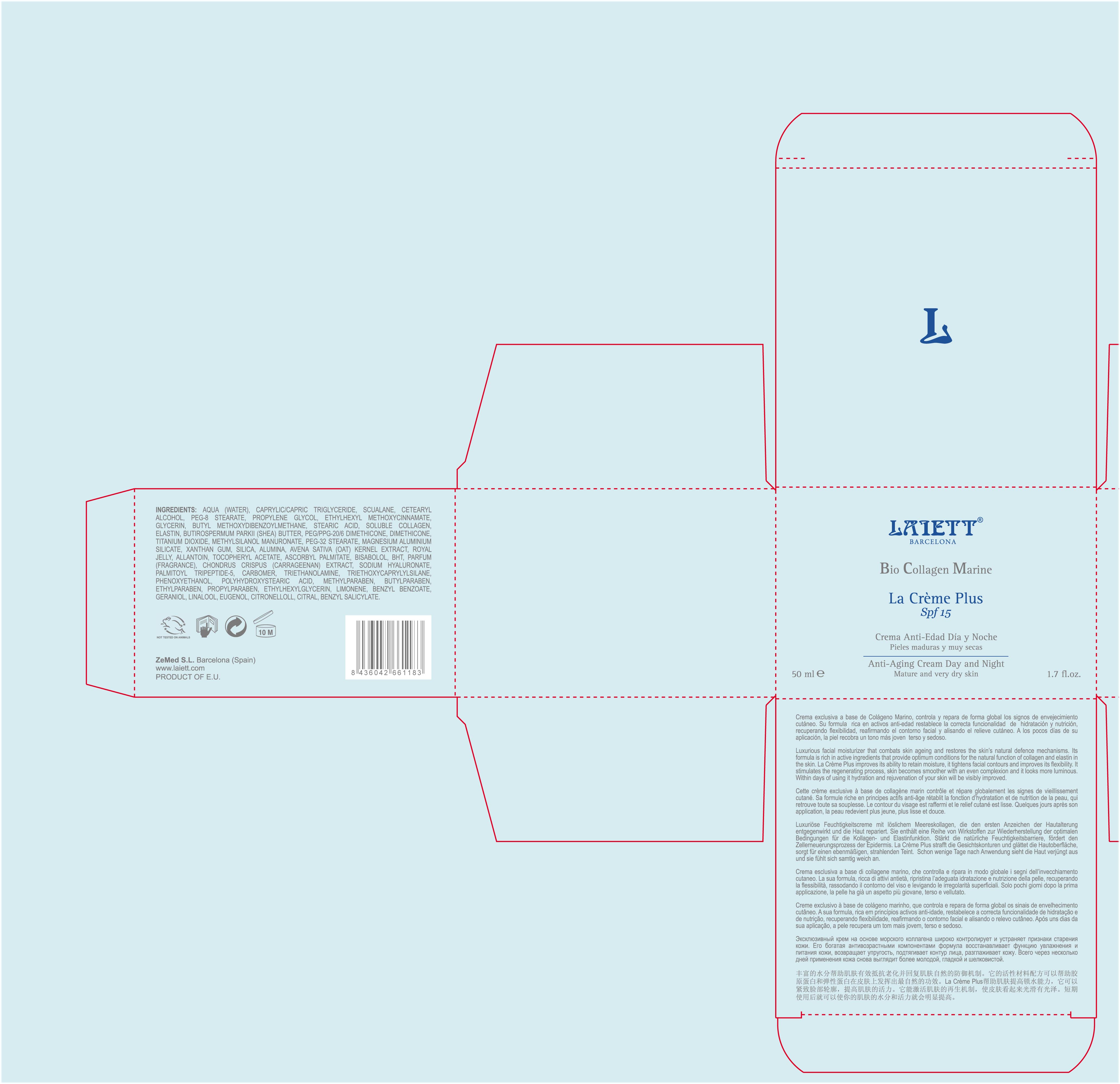
Luxurious facial moisturizer that combats skin ageing and ensures that skin is well nourished and moisturized round-the clock. Its formula is rich in active ingredients that help provide optimum conditions for the natural function of collagen and elastin in the skin. La Creme helps reduce expression lines and wrinkles and improves the skin's flexibility and elasticity. Skin looks firmer and more luminous. Within days of using it there will be a visible improvement.
50ml. 1.7fl. oz.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LA CREME P
octinoxate avobenzone titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:42248-133 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 3.75 mL in 50 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 1.5 mL in 50 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 12.5 mL in 50 mL Inactive Ingredients Ingredient Name Strength MAGNESIUM ALUMINUM SILICATE (UNII: 6M3P64V0NC) PROPYLPARABEN (UNII: Z8IX2SC1OH) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) DIMETHICONE (UNII: 92RU3N3Y1O) XANTHAN GUM (UNII: TTV12P4NEE) SHEA BUTTER (UNII: K49155WL9Y) WATER (UNII: 059QF0KO0R) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) TRICAPRYLIN (UNII: 6P92858988) PEG-8 STEARATE (UNII: 2P9L47VI5E) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CHONDRUS CRISPUS (UNII: OQS23HUA1X) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) ASCORBYL PALMITATE (UNII: QN83US2B0N) CARBOMER 934 (UNII: Z135WT9208) ROYAL JELLY (UNII: L497I37F0C) AVENA SATIVA FLOWERING TOP (UNII: MA9CQJ3F7F) METHYLPARABEN (UNII: A2I8C7HI9T) COLLAGEN, SOLUBLE, FISH SKIN (UNII: 8JC99XGU4W) PALMITOYL TRIPEPTIDE-5 (UNII: 2A3916MQHO) TROLAMINE (UNII: 9O3K93S3TK) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) STEARIC ACID (UNII: 4ELV7Z65AP) LEVOMENOL (UNII: 24WE03BX2T) ALLANTOIN (UNII: 344S277G0Z) BUTYLPARABEN (UNII: 3QPI1U3FV8) PEG-32 STEARATE (UNII: 33GX5WQC0M) ETHYLPARABEN (UNII: 14255EXE39) GLYCERIN (UNII: PDC6A3C0OX) SQUALENE (UNII: 7QWM220FJH) BENZYL BENZOATE (UNII: N863NB338G) HYALURONATE SODIUM (UNII: YSE9PPT4TH) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) LIMONENE, (+/-)- (UNII: 9MC3I34447) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) CITRAL (UNII: T7EU0O9VPP) PHENOXYETHANOL (UNII: HIE492ZZ3T) GERANIOL (UNII: L837108USY) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) LINALOOL, (+/-)- (UNII: D81QY6I88E) EUGENOL (UNII: 3T8H1794QW) BENZYL SALICYLATE (UNII: WAO5MNK9TU) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42248-133-02 1 in 1 BOX 1 50 mL in 1 JAR Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 12/01/2011 Labeler - Zenith Medicosm SL (464239694) Registrant - Zenith Medicosm SL (464239694) Establishment Name Address ID/FEI Business Operations Zenith Medicosm SL 464239694 manufacture